Question
1 mole of an ideal gas is used in a Carnot cycle. The maximum pressure (P1) and temperature (TH) are 1.23 atm and 300K, respectively.
1 mole of an ideal gas is used in a Carnot cycle. The maximum pressure (P1) and temperature (TH) are 1.23 atm and 300K, respectively. The ratio of isothermal expansion is V2/V1 = 2, and the ratio of adiabatic compression is V4/V1 = 1.8. Assuming that the gas initially occupies a volume of 20 L, calculate the following ( = 5/3): a) The minimum temperature (Tc) in the cycle. b) The pressure values at each point in the cycle (in bar). c) The efficiency of the heat engine. Draw a schematic diagram of the heat engine. d) The amount of heat transferred from the hot heat source to the heat engine (in calories). 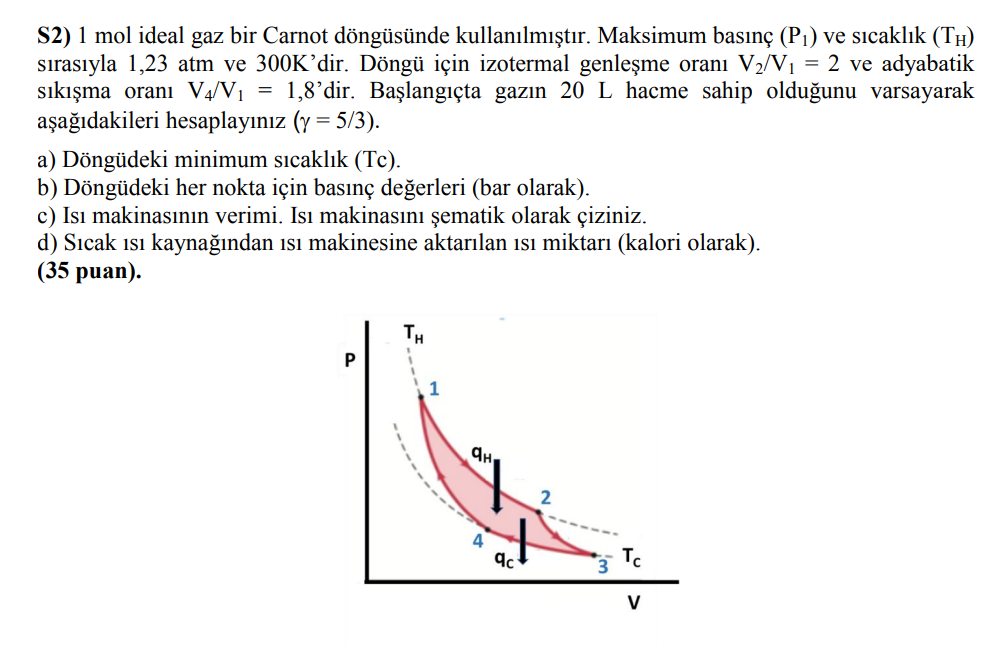
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


