Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene (B) At 40C and 120kPa absolute. In the process, dichlorobenzene (D) is
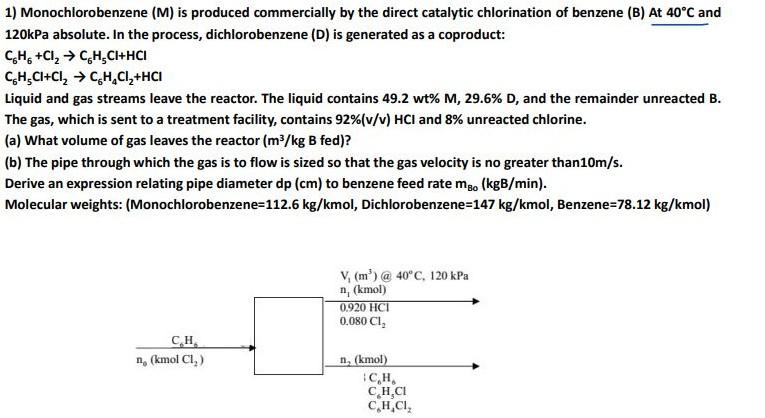
1) Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene (B) At 40C and 120kPa absolute. In the process, dichlorobenzene (D) is generated as a coproduct: C6H6+Cl2C6H5Cl+HClC6H5Cl+Cl2C6H4Cl2+HCl Liquid and gas streams leave the reactor. The liquid contains 49.2wt%M,29.6%D, and the remainder unreacted B. The gas, which is sent to a treatment facility, contains 92%(v/v)HCl and 8% unreacted chlorine. (a) What volume of gas leaves the reactor (m3/kgB fed)? (b) The pipe through which the gas is to flow is sized so that the gas velocity is no greater than 10m/s. Derive an expression relating pipe diameter dp(cm) to benzene feed rate mBo(kgB/min). Molecular weights: (Monochlorobenzene=112.6 kg/ kmol, Dichlorobenzene=147 kg /kmol, Benzene=78.12 kg/ kmol )
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


