Answered step by step
Verified Expert Solution
Question
1 Approved Answer
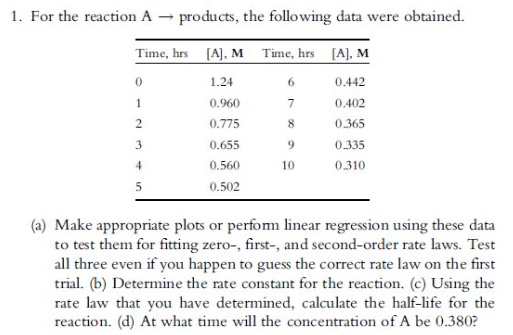
1 . Para los productos de reacci n A - > , se obtuvieron los siguientes datos . Haga gr ficos apropiados o realice una
Para los productos de reaccin A se obtuvieron los siguientesdatos Haga grficos apropiados o realice una regresin lineal utilizando estos datos para probarlos para que se ajusten a las leyes de tasa cero, de primer y segundo orden. Pruebe los tres incluso si adivina la ley de velocidad correcta en el primer ensayo. b Determine la constante de velocidad para la reaccinc Usando la ley de velocidad que ha determinado, calcule la vida media de la reaccindA qu momento la concentracin de AserdeFor the reaction products, the following data were obtained.
tableTime hrsAMTime, hrsAM
a Make appropriate plots or perform linear regression using these data to test them for fitting zero first and secondorder rate laws. Test all three even if you happen to guess the correct rate law on the first trial. b Determine the rate constant for the reaction. c Using the rate law that you have determined, calculate the halflife for the reaction. d At what time will the concentration of A be
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


