Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 please answer all parts of the question An automobile engine provides 583 Joules of work to push the pistons and generates 2279 Joules of
1 please answer all parts of the question 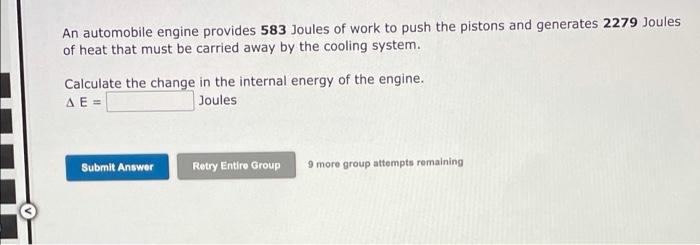

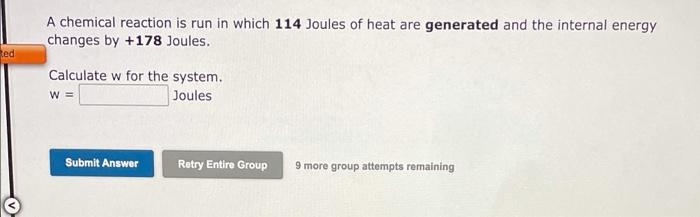
An automobile engine provides 583 Joules of work to push the pistons and generates 2279 Joules of heat that must be carried away by the cooling system. Calculate the change in the internal energy of the engine. A E = Joules Submit Answer Retry Entire Group 9 more group attempts remaining A chemical reaction is run in which 214 Joules of heat are absorbed and 787 Joules of work are done on the system. Calculate the change in the internal energy of the chemical system. = Joules Submit Answer Retry Entire Group 9 more group attempts remaining A chemical reaction is run in which 114 Joules of heat are generated and the internal energy changes by +178 Joules. Red Calculate w for the system. W = Joules Submit Answer Retry Entire Group 9 more group attempts remaining 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


