Answered step by step
Verified Expert Solution
Question
1 Approved Answer
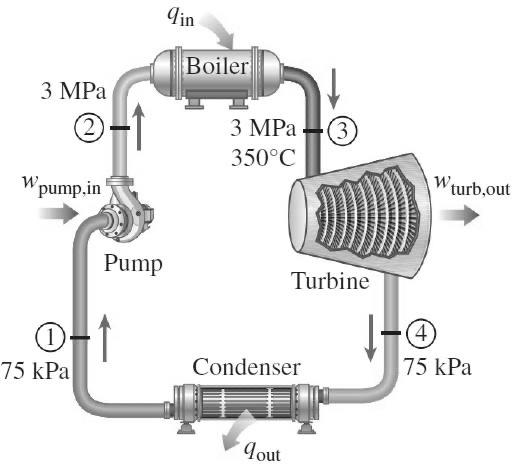
1. Rankine cycle A steam power plant operates on the simple ideal Rankine cycle. The series of processes are illustrated in the following figure. The
1. Rankine cycle
A steam power plant operates on the simple ideal Rankine cycle.
The series of processes are illustrated in the following figure.
The superheated steam entering the turbine has a pressure of 3 Mpa and a temperature of 350C (stream 3 in the figure). In the turbine expansion occurs, so the pressure drops to 75 kPa and some of the steam will melt (flow 4 in the figure). This vapor-liquid mixture is then liquefied in the condenser so that it turns into a saturated liquid with a pressure of 75 kPa (stream 1 in the figure). The saturated liquid is then pumped so that the pressure rises to 3 MPa (stream 2 in the figure), then fed to the boiler where it will be heated until it becomes superheated steam again.
Given: h2 = 387.47 kJ/kg ; h4 = 2403.0 kJ/kg
A. Determine the enthalpy and entropy of the flow entering the pump and the flow entering the turbine!
B. Explain what is meant by entropy! Also explain what is the relationship between entropy and evaporation
of a liquid!
C. The expansion process in the turbine is ideally an isentropic process, which means that the entropy of the flow entering the turbine is equal to the enthalpy of the flow leaving the turbine. Determine what fraction of steam is at the turbine outlet.
D. Calculate the thermal efficiency of the Rankine cycle using your answer to question 1a and the data given in the problem.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


