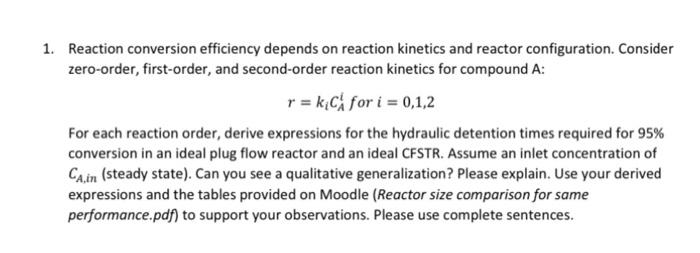
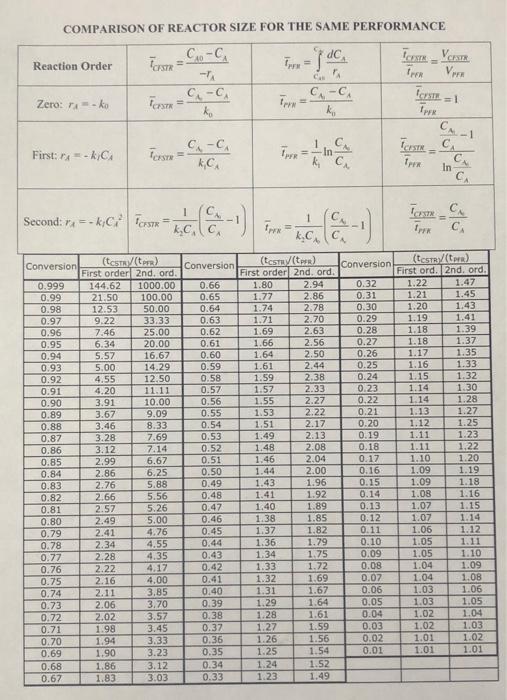
1. Reaction conversion efficiency depends on reaction kinetics and reactor configuration. Consider zero-order, first-order, and second-order reaction kinetics for compound A: r = k C for i = 0,1,2 For each reaction order, derive expressions for the hydraulic detention times required for 95% conversion in an ideal plug flow reactor and an ideal CFSTR. Assume an inlet concentration of Ca.in (steady state). Can you see a qualitative generalization? Please explain. Use your derived expressions and the tables provided on Moodle (Reactor size comparison for same performance.pdf) to support your observations. Please use complete sentences. COMPARISON OF REACTOR SIZE FOR THE SAME PERFORMANCE .. - CA Tos Reaction Order dc im Tron V C-CA TOISE=1 Zero: Ako C.-C. ko Fr= px 1 TCNSTRC First: A=-kC C.-C. , Tersru k", Terk Second: =-kC Prste= k.c - - - 1 TC 0 x = .. Conversion 1.27 Conversion! (test) Conversion (testy (tr) First order 2nd, ord. First order 2nd. ord. 0.999 144.62 1000.00 0.66 1.80 2.94 0.99 21.50 100.00 0.65 1.77 2.86 0.98 12.53 50.00 0.64 1.74 2.78 0.97 9.22 33.33 0.63 1.71 2.70 0.96 7.46 25.00 0.62 1.69 2.63 0.95 6.34 20.00 0.61 1.66 2.56 0.94 5.57 16.67 0.60 1.64 2.50 0.93 5.00 14.29 0.59 1.61 2.44 0.92 4.55 12.50 0.58 1.59 2.38 0.91 4.20 11.11 0.57 1.57 2.33 0.90 3.91 10.00 0.56 1.55 2.27 0.89 3.67 9.09 0.55 1.53 2.22 0.88 3.46 8.33 0.54 1.51 2.17 0.87 3.28 7.69 0.53 1.49 2.13 0.86 3.12 7.14 0.52 1.48 2.08 0.85 2.99 6.67 0.51 1.46 2.04 0.84 2.86 6.25 0.50 1.44 2.00 0.83 2.76 5.88 0.49 1.43 1.96 0.82 2.66 5.56 0.48 1.41 1.92 0.81 2.57 5.26 0.47 1.40 1.89 0.80 2.49 5.00 0.46 1.38 1.85 0.79 2.41 4.76 0.45 1.37 1.82 0.78 2.34 4.55 0.44 1.36 1.79 0.77 2.28 4.35 0.43 1.34 1.75 0.76 2.22 4.17 0.42 1.33 1.72 0.75 2.16 4.00 0.41 1.32 1.69 0.74 2.11 3.85 0.40 1.31 1.67 0.73 2.06 3.70 0.39 1.29 1.64 0.72 2.02 3.57 0.38 1.28 1.61 0.71 1.98 3.45 0.37 1.27 1.59 0.70 1.94 3.33 0.36 1.26 1.56 0.69 1.90 3.23 0.35 1.25 0.68 1.86 3.12 0.34 1.24 1.52 0.67 1.83 3.03 0.33 1.23 1.49 0.32 0.31 0.30 0.29 0.28 0.27 0.26 0.25 0.24 0.23 0.22 0.21 0.20 0.19 0.18 0.17 0.16 0.15 0.14 0.13 0.12 0.11 0.10 0.09 0.08 0.07 0.06 0.05 0.04 0.03 0.02 0.01 (tests:/(ter) First ord. 2nd. ord. 1.22 1.47 1.21 1.45 1.20 1.43 1.19 1.41 1.18 1.39 1.18 1.37 1.17 1.35 1.16 1.33 1.15 1.32 1.14 1.30 1.14 1.28 1.13 1.12 1.25 1.11 1.23 1.11 1.22 1.10 1.20 1.09 1.19 1.09 1.18 1.08 1.16 1.07 1.15 1.07 1.14 1.06 1.12 1.05 1.11 1.05 1.10 1.04 1.09 1.04 1.08 1.03 1.06 1.03 1.05 1.02 1.04 1.02 1.03 1.01 1.02 1.01 1.01 1.54