Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Sakura is a chemist assigned to monitor the water hardness of treated water samples by complexometric titration. a. First, she dissolved 0.1490g of CaCO3(MW=100.09gmol1)
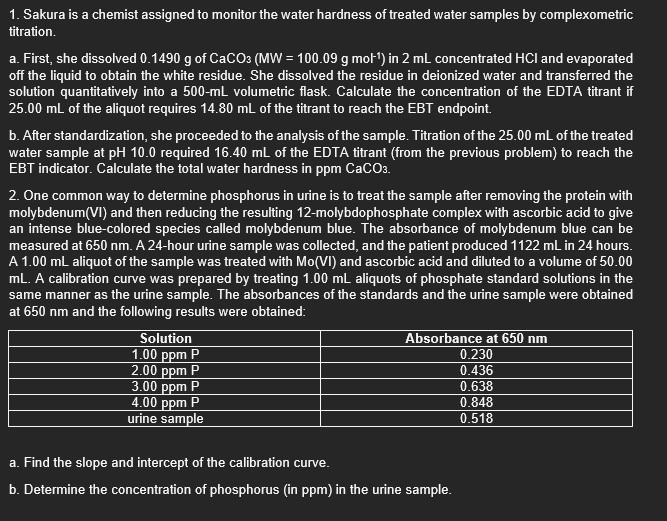
1. Sakura is a chemist assigned to monitor the water hardness of treated water samples by complexometric titration. a. First, she dissolved 0.1490g of CaCO3(MW=100.09gmol1) in 2mL concentrated HCl and evaporated off the liquid to obtain the white residue. She dissolved the residue in deionized water and transferred the solution quantitatively into a 500mL volumetric flask. Calculate the concentration of the EDTA titrant if 25.00mL of the aliquot requires 14.80mL of the titrant to reach the EB endpoint. b. After standardization, she proceeded to the analysis of the sample. Titration of the 25.00mL of the treated water sample at pH10.0 required 16.40mL of the EDTA titrant (from the previous problem) to reach the EBT indicator. Calculate the total water hardness in ppmCaCO3. 2. One common way to determine phosphorus in urine is to treat the sample after removing the protein with molybdenum(VI) and then reducing the resulting 12-molybdophosphate complex with ascorbic acid to give an intense blue-colored species called molybdenum blue. The absorbance of molybdenum blue can be measured at 650nm. A 24-hour urine sample was collected, and the patient produced 1122mL in 24 hours. A 1.00mL aliquot of the sample was treated with Mo(VI) and ascorbic acid and diluted to a volume of 50.00 mL. A calibration curve was prepared by treating 1.00mL aliquots of phosphate standard solutions in the same manner as the urine sample. The absorbances of the standards and the urine sample were obtained at 650nm and the following results were obtained: a. Find the slope and intercept of the callbration curve. b. Determine the concentration of phosphorus (in ppm) in the urine sample
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


