Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1). Select the net ionic equation for the reaction that occurs when sodium hydroxide and nickel(II) nitrate are mixed. No reaction occurs 2 Na+(aq) +
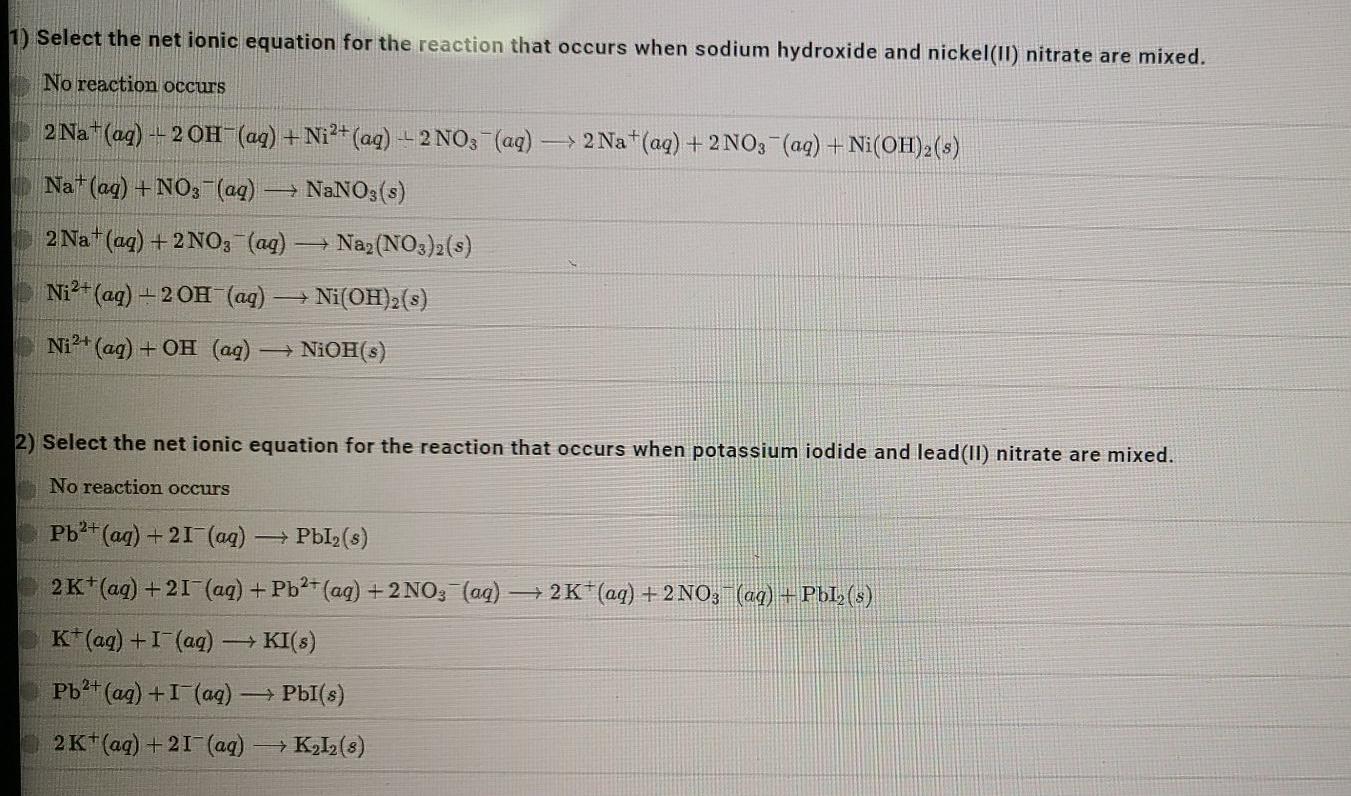
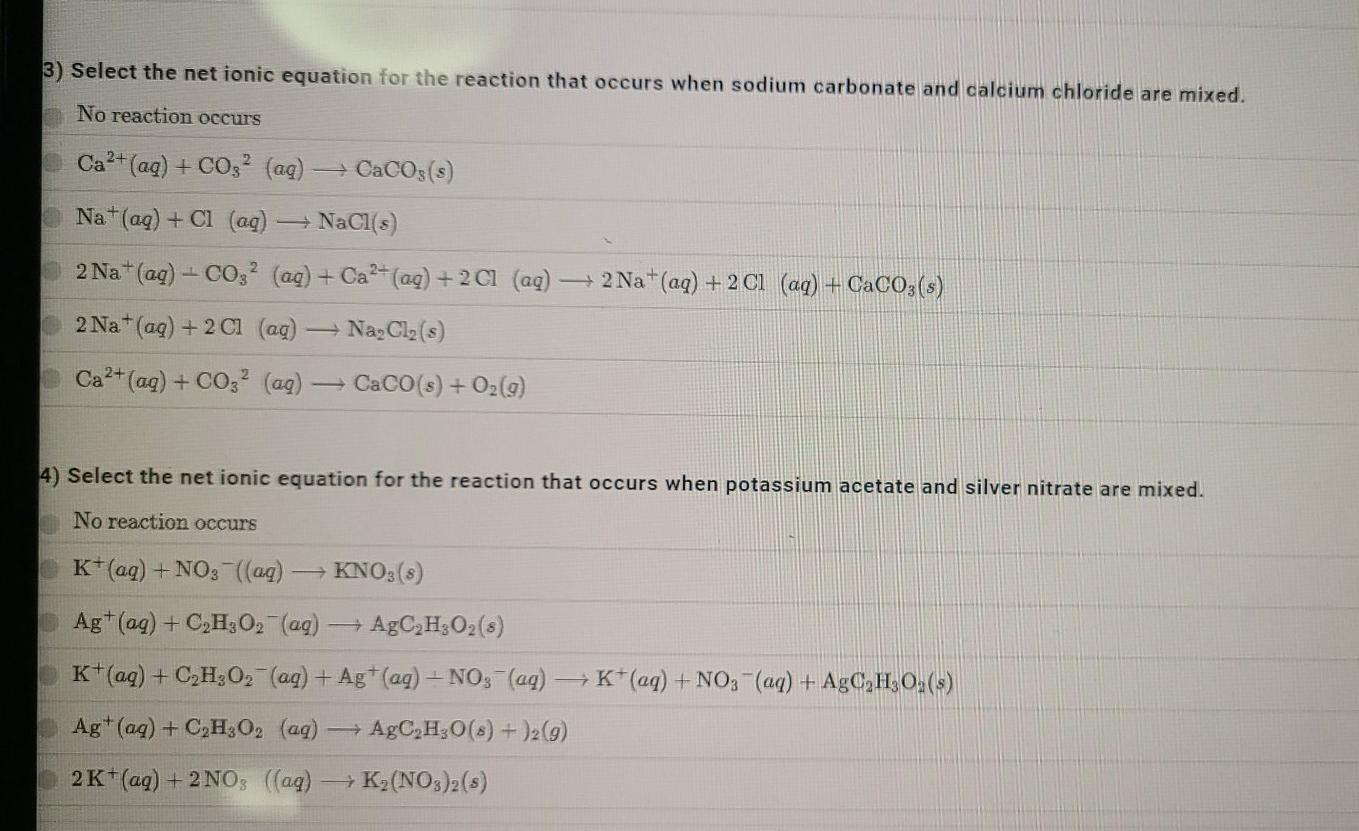
1). Select the net ionic equation for the reaction that occurs when sodium hydroxide and nickel(II) nitrate are mixed. No reaction occurs 2 Na+(aq) + 2 OH-(aq) + Ni2+ (aq) + 2NO3 -(aq) - -> 2Na+(aq) + 2NO3(aq) + Ni(OH)2(s) Nat(aq) + NO3- (aq) - NaNO3(s) 2 Na+ (aq) + 2NO3(aq) Na2(NO3)2(s) Ni2+ (aq) + 2OH- (aq) Ni(OH)2(3) Ni2+ (aq) + OH (aq) NiOH(3) 2) Select the net ionic equation for the reaction that occurs when potassium iodide and lead(II) nitrate are mixed. No reaction occurs Pb2+ (aq) +21 (aq) PbI(s) 2 K+(aq) +27 (aq) + Pb2+ (aq) + 2NO3(aq) + 2K+(aq) + 2NO3- (aq) + PbL,(s) K*(aq) + I (aq) KI(8) Pb2+ (aq) +1 (aq) PbI(s) 2K+(aq) +21 (aq) + K2I2(8) 3) Select the net ionic equation for the reaction that occurs when sodium carbonate and calcium chloride are mixed. No reaction occurs Ca2+ (aq) + CO2 (aq) = CaCO3(s) Na+(aq) + Cl (aq) NaCl(s) 2 Na+ (aq) + CO2 (aq) + Ca2+ (aq) + 21 (aq) +2Na+(aq) + 2 C1 (aq) + CaCO3(s) 2 Na+ (aq) + 2 C1 (aq) Na Cl (5) Ca2+ (aq) + CO32 (ag) CaCO(s) + O2(g) 4) Select the net ionic equation for the reaction that occurs when potassium acetate and silver nitrate are mixed. No reaction occurs K+(aq) + NO3(aq) KNO3 (8) Ag+ (aq) + C,H,O2 (aq) AgC H302 (8) K+ (aq) + C,H,O2 (aq) + Ag+ (aq) + NO3(aq) + K+ (aq) + NO3(aq) + AgC H30,(s) Ag+ (aq) + C,H,O2 (aq) AgC H30(8) + 12 (9) 2K+ (aq) + 2NO3 ((aq) K (NO3)2(s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


