Question
1. Solve for the following electrochemical cell: Positive electrode: Pb 2+ + 2e- Pb Negative electrode: PbSO 4 + 2e- Pb + SO 4 2-
1. Solve for the following electrochemical cell:
Positive electrode: Pb2+ + 2e- Pb
Negative electrode: PbSO4 + 2e- Pb + SO42-
(a) Determine the electrode potentials at standard conditions and calculate corresponding cell voltage. Assume that the standard electrode potential is shown by the table below
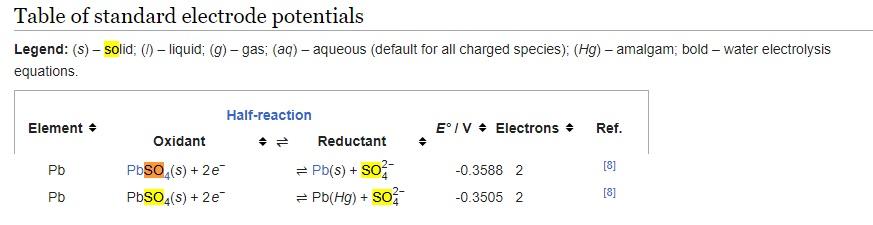
(b) What is the dependence of cell voltage on the concentration of Pb2+ and SO42- in the solution?
(c) If the activities of pure solids are 1, and the solubility product is defined as [Pb2+][SO42-]. Calculate the solubility product.
(d) Recalculate the solubility product at 80 degrees C, and if the voltage of the cell has a temperature coefficient of -1 mV/K.
Table of standard electrode potentials Legend: (s) - solid; (l) - liquid; (g) - gas; (aq) - aqueous (default for all charged species); (Hg) - amalgam; bold - water electrolysis equationsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


