Question
1. Starting with diethyl malonate, the generic synthesis of barbituric acid derivatives is shown below. Please explain how Veronal (barbital) is formed, which is the
1. Starting with diethyl malonate, the generic synthesis of barbituric acid derivatives is shown below. Please explain how Veronal (barbital) is formed, which is the derivative in which both R and R' are ethyl groups. Make sure you have an appropriate base and alkyl halide on hand. You can also suppose that you will neutralise the solution in the end with a source of H+.
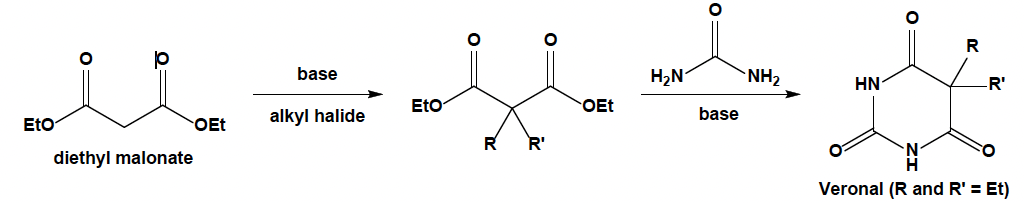
2. The derivative phenobarbital, in which the R and R' groups are phenyl and ethyl, cannot be produced in the same way as barbital. Explain why the same strategy cannot be used based on your mechanism from the previous question. HINT: Consider the variables that impact SN2 reactions.
3. How is phenobarbital created? Cite your sources and provide a synthesised strategy.
alkylhalidebase diethyl malonate Veronal (R and R=Et )Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


