Question: 1. Starting with the equation (11.75) for excess Gibbs free energy for the case of the Margules single parameter model, use the relationship between activity
1. Starting with the equation (11.75) for excess Gibbs free energy for the case of the Margules single
parameter model, use the relationship between activity coefficient and excess free energy (11.73) to
derive the equations (11.81 and 11.82) for the Margules single parameter activity coefficient model.
2. Starting with the Wilson equations, determine values or expressions for the activity coefficient at infinite
dilution for each of components 1 and 2. Explain how the model parameters can be determined from an
experimentally measured P-x-y data set?
3. Starting with the Van Laar equations, determine values or expressions for the activity coefficient at
infinite dilution for each of components 1 and 2. Explain how the model parameters can be determined
from an experimentally measured P-x-y data set?
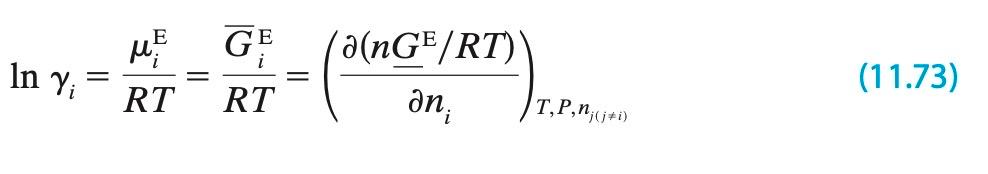



E GE In Yi = ME i = a(nGE/RT)\ (11.73) T,P,nj(j+i) RT RT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


