Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Steam is compressed from 10 bar and 673K to an exit temperature of 873K. If the isentropic efficiency is 75%, calculate the power requirements
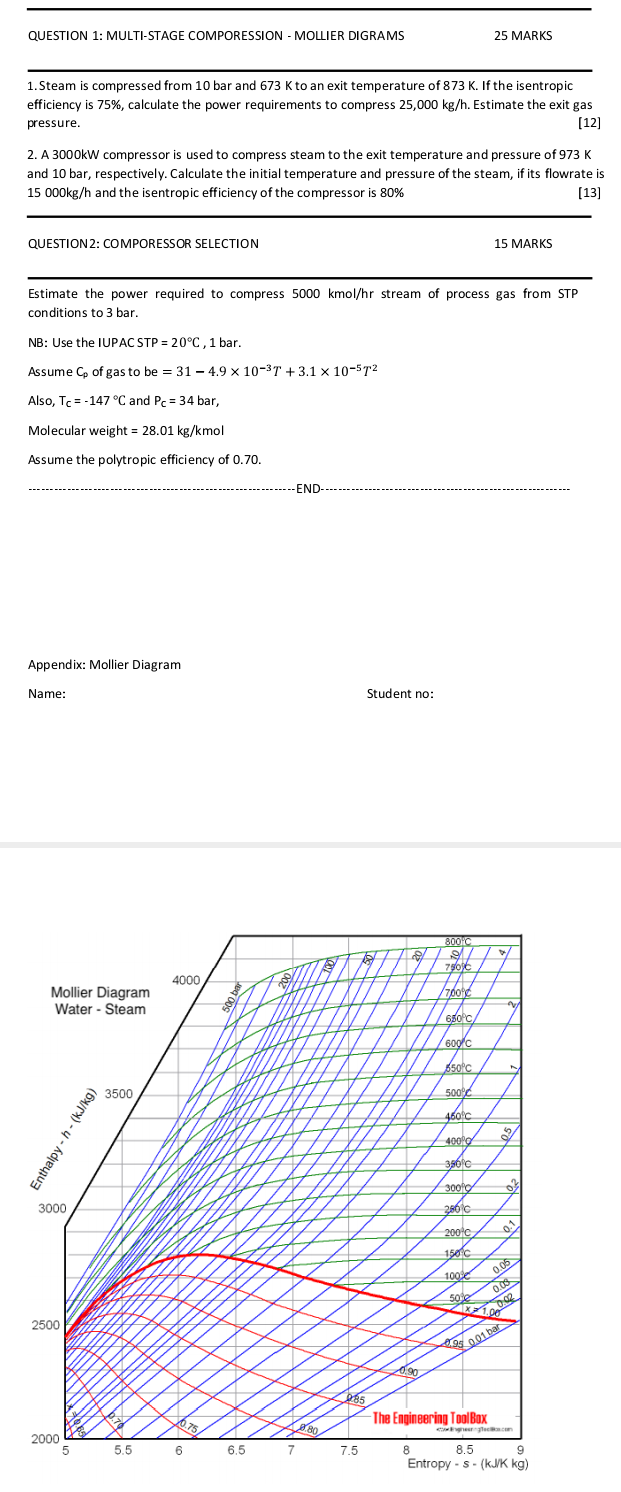
1. Steam is compressed from 10 bar and 673K to an exit temperature of 873K. If the isentropic efficiency is 75%, calculate the power requirements to compress 25,000kg/h. Estimate the exit gas pressure. [12] 2. A 3000kW compressor is used to compress steam to the exit temperature and pressure of 973K and 10 bar, respectively. Calculate the initial temperature and pressure of the steam, if its flowrate is 15000kg/h and the isentropic efficiency of the compressor is 80% [13] QUESTION 2: COMPORESSOR SELECTION 15 MARKS Estimate the power required to compress 5000kmol/hr stream of process gas from STP conditions to 3 bar. NB: Use the IUPAC STP =20C,1 bar. Assume Cp of gas to be =314.9103T+3.1105T2 Also, TC=147C and PC=34 bar, Molecular weight =28.01kg/kmol Assume the polytropic efficiency of 0.70
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


