Question
1. The C-NMR spectrum of triphenylmethanol shows three peaks inthe 120 - 165 ppm range and one peak at 82.3 ppm, however theunusally tall peak
1. The C-NMR spectrum of triphenylmethanol shows three peaks inthe 120 - 165 ppm range and one peak at 82.3 ppm, however theunusally tall peak at 127.9 ppm is a very rare case of twooverlapped peaks that are so exactly overlapped they are seen asone. Draw the structure of triphenylmethanol showing which C's areequivalent and which are inequivalent and indicate the plane ofsymmetry (or rotational exchanges) that exchange equivalent C's inthe molecule. Which carbon in triphenylmethanol causes the peak at82.3 ppm?
2. Does the C-NMR spectrum of the below spectrum show product?Is it pure? Are there any unreacted reactants, side products orsolvents present?
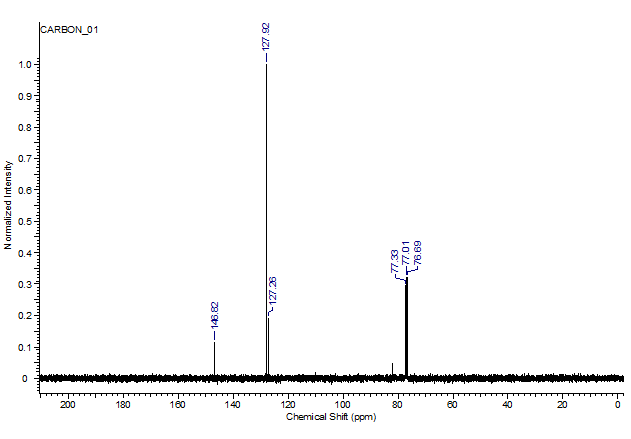
Normalized Intensity 1.0- 0.9 0.8- 0.7- 0.6- 0.5- 0.4- 0.35 0.2- 0.1- 0 CARBON_01 200 180 160 -145.82 140 -127.92 127.26 Chemical Shift (ppm) 77.33 77.01 76.69 80 8 60 40 20
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
Answer 4 13 C peaks a 823 ppm b d1466 PPM d OH The 4 1272 ppm C 1279 ppm ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


