Question
1-Draw the product of the complete hydrogenation of ethyne. 2-Draw the product of the hydration of 2-butene. 3-Draw the molecule resulting from the addition of
1-Draw the product of the complete hydrogenation of ethyne.
2-Draw the product of the hydration of 2-butene.
3-Draw the molecule resulting from the addition of HBr topropene.
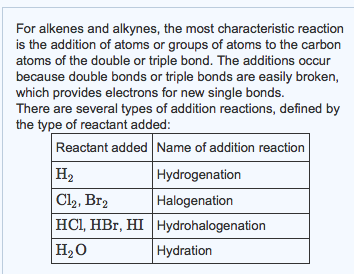
For alkenes and alkynes, the most characteristic reaction is the addition of atoms or groups of atoms to the carbon atoms of the double or triple bond. The additions occur because double bonds or triple bonds are easily broken, which provides electrons for new single bonds. There are several types of addition reactions, defined by the type of reactant added: Reactant added Name of addition reaction H Cl2, Br2 HCl, HBr, HI Hydrohalogenation HO Hydration Hydrogenation Halogenation
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
10th Edition
978-1305957404, 9781305957404
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



