Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The density of CO2(MA=44.0g/mol) at STP is 1.98kg/m3. Under these conditions, the solubility of CO2 in water is 0.1449grams of CO2/100mL of H2O. The
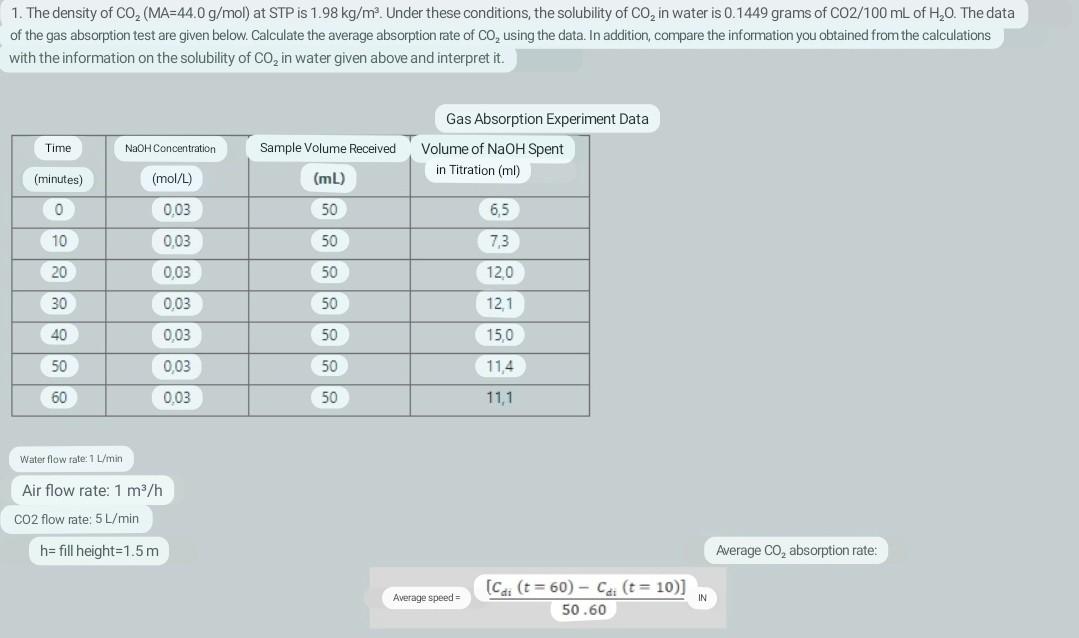
1. The density of CO2(MA=44.0g/mol) at STP is 1.98kg/m3. Under these conditions, the solubility of CO2 in water is 0.1449grams of CO2/100mL of H2O. The data of the gas absorption test are given below. Calculate the average absorption rate of CO2 using the data. In addition, compare the information you obtained from the calculations with the information on the solubility of CO2 in water given above and interpret it. [Cdi(t=60)Cdi(t=10)]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


