Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The density of liquid gallium is 6.09 g.cm at 35C. If this element is employed in a barometer instead of mercury, what is
![]()
![]()

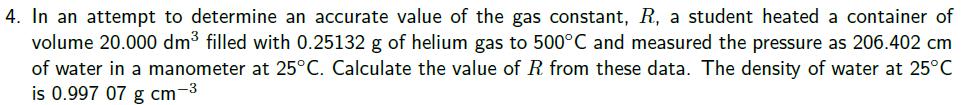
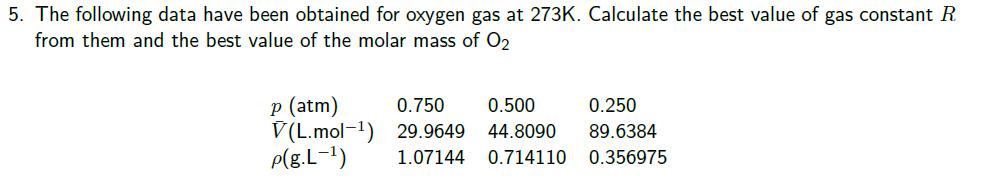
1. The density of liquid gallium is 6.09 g.cm at 35C. If this element is employed in a barometer instead of mercury, what is the height of a column of gallium sustained in the barometer at 1 atm. pressure? 2. Find the molar mass of a gas whose density is 1.80 g. L-1 at 25C and 880 torr. 3. A car tyre was inflated to a pressure of 24 lb in 2 (1.00 atm = 14.7 lb in-2) on a winter's day when the temperature was -5C. What pressure will be found, assuming no leaks have occurred and that the volume is constant, on a subsequent summer's day when the temperature is 35C? 4. In an attempt to determine an accurate value of the gas constant, R, a student heated a container of volume 20.000 dm filled with 0.25132 g of helium gas to 500C and measured the pressure as 206.402 cm of water in a manometer at 25C. Calculate the value of R from these data. The density of water at 25C is 0.997 07 g cm-3 5. The following data have been obtained for oxygen gas at 273K. Calculate the best value of gas constant R from them and the best value of the molar mass of O2 p (atm) 0.750 V(L.mol-1) 29.9649 0.500 0.250 44.8090 89.6384 p(g.L-1) 1.07144 0.714110 0.356975
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


