Question
1. The diagram below shows the bonding between aluminium chloride and ammonia. (a)Name the types of bonds that exist in the molecule.(1 mark) (b)How many
1. The diagram below shows the bonding between aluminium chloride and ammonia.
(a)Name the types of bonds that exist in the molecule.(1 mark)
(b)How many electrons are used for bonding in the molecule?
2. Ammonium ion has the following structure:
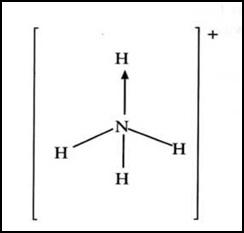
Label on the structure:
(a)Covalent bond; (1 mark)
(b)Coordinate (dative) bond.
3. Use the following information on substances S, T and hydrogen to answer the questions that follow:
I.T displaces V from a solution containing V ions
II.hydrogen reacts with the heated oxide of S but has no effects on heated oxide of V.
(a)Arrange substances S, t, V and hydrogen in the order of increasing reactivity.(2 marks)
(b)If T and V are divalent metals, write an ionic equation for the reaction in (i) above. (1 mark)
H H H H
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


