Question
The grid below is part of the periodic table. Use it to answer the questions that follow. (the letters are not the actual symbols of
The grid below is part of the periodic table. Use it to answer the questions that follow. (the letters are not the actual symbols of the elements).
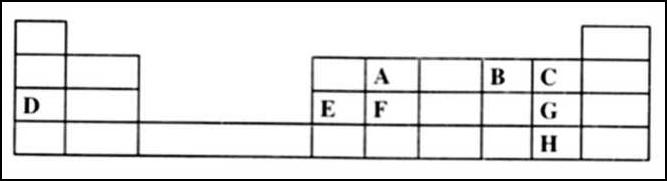
(a)Which is the most reactive non-metallic element shown in the table? Explain. (2 marks)
(b)(i) Write the formula of the compound formed when element A reacts with element B(1 mark)
(ii) Name the bond type in the compound formed in b(i) above (1 mark)
(c)(i) What is the name given to the group of elements where, C, G and H belong?
(ii) Write an equation for the reaction that occurs when C in gaseous form is passed through a solution containing ions of element H. (1 mark)
(d)The melting points of elements F and G are 1410 °C and -101 °C respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of F and G. (2 marks)
(e)D forms two oxides. Write the formula of each of the two oxides. (1 mark)
(f)J is an element that belongs to the 3rd period of the periodic table and a member of the alkaline earth elements. Show the position of J in the grid (1 mark)
A B C D E F G H
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


