Question
1. The following cell notation represents the electrochemical cell setup during experiment. [EHg2Cl|Hg|CI = 0.2415V, EAgCl|AgCl = 0.2224V,EAg|Ag+ = -0.80V] Ag (s) | AgCl
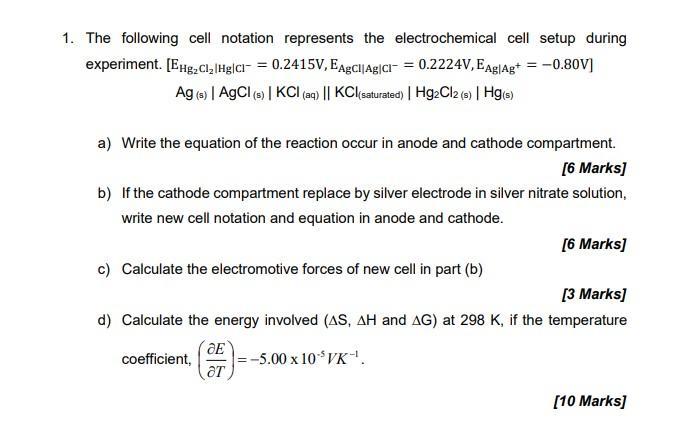
1. The following cell notation represents the electrochemical cell setup during experiment. [EHg2Cl|Hg|CI = 0.2415V, EAgCl|AgCl = 0.2224V,EAg|Ag+ = -0.80V] Ag (s) | AgCl (s) | KCI (aq) || KCl (saturated) | Hg2Cl2 (5) | Hg(s) a) Write the equation of the reaction occur in anode and cathode compartment. [6 Marks] b) If the cathode compartment replace by silver electrode in silver nitrate solution, write new cell notation and equation in anode and cathode. [6 Marks] c) Calculate the electromotive forces of new cell in part (b) [3 Marks] d) Calculate the energy involved (AS, AH and AG) at 298 K, if the temperature coefficient, |=5.00 x 10$VK. [10 Marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry Principles And Modern Applications
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
11th Edition
0132931281, 978-0132931281
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



