Question
1. The oxidation of SO2 to SO3 is catalyzed by NO2. The reaction proceeds as follows: a. Write the balanced equation for the overall oxidation
1. The oxidation of SO2 to SO3 is catalyzed by NO2. The reaction proceeds as follows:
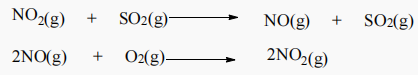
a. Write the balanced equation for the overall oxidation reaction.
b. Why do we consider NO2 as a catalyst and not an intermediate in the reaction?
c. Is this an example of homogeneous catalysis or heterogeneous catalysis? Explain your answer.
2. The following mechanism has been proposed for the gas phase reaction of H2 with ICI:
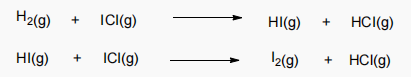
a. Write the balanced equation for the overall reaction.
b. Identify the intermediate in the mechanism.
c. Write rate laws for each elementary step in the mechanism and specify the molecularity.
d. If the first step is slow and the second step is fast, what rate law do you expect to be observed for the overall reaction?
NO2(g)+SO2(g)NO(g)+SO2(g)2NO(g)+O2(g)2NO2(g) H2(g)+ICl(g)HI(g)+HCl(g)HI(g)+ICl(g)I2(g)+HCl(g)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


