Answered step by step
Verified Expert Solution
Question
1 Approved Answer
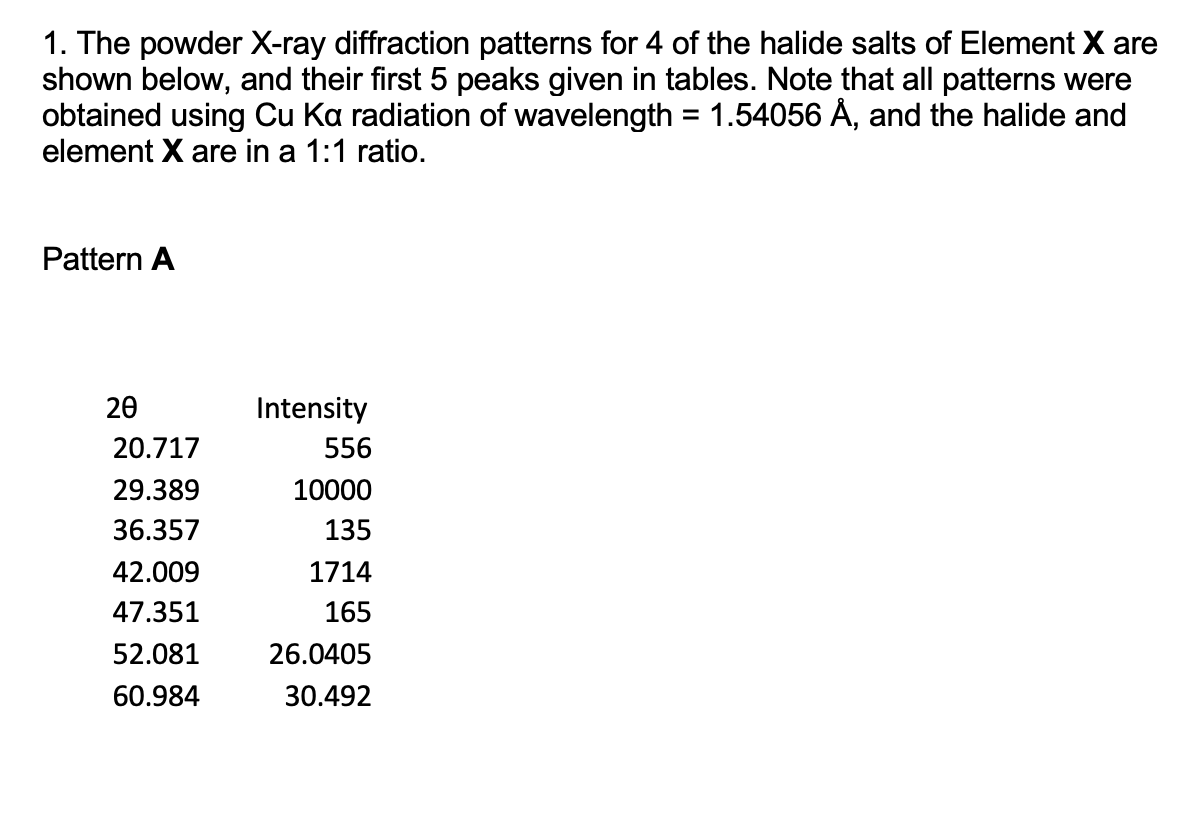
? ? ? 1. The powder X-ray diffraction patterns for 4 of the halide salts of Element X are shown below, and their first 5
 ?
? ?
? ?
?
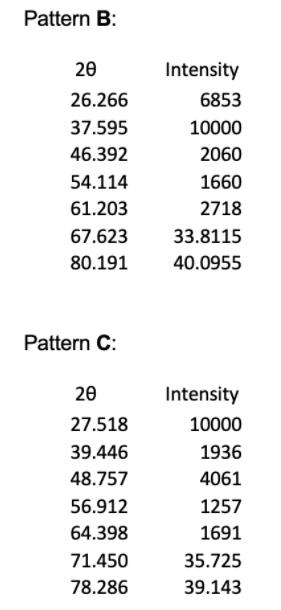
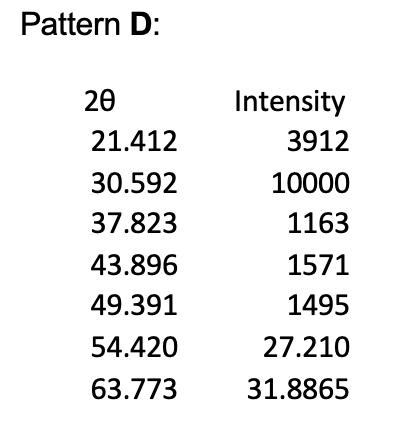
1. The powder X-ray diffraction patterns for 4 of the halide salts of Element X are shown below, and their first 5 peaks given in tables. Note that all patterns were obtained using Cu Ka radiation of wavelength = 1.54056 , and the halide and element X are in a 1:1 ratio. Pattern A 20 20.717 29.389 36.357 42.009 47.351 52.081 60.984 Intensity 556 10000 135 1714 165 26.0405 30.492 Pattern B: 20 26.266 37.595 46.392 54.114 61.203 67.623 80.191 Pattern C: 20 27.518 39.446 48.757 56.912 64.398 71.450 78.286 Intensity 6853 10000 2060 1660 2718 33.8115 40.0955 Intensity 10000 1936 4061 1257 1691 35.725 39.143 Pattern D: 20 21.412 30.592 37.823 43.896 49.391 54.420 63.773 Intensity 3912 10000 1163 1571 1495 27.210 31.8865 (e) (i) Given the ionic radius of element X is 181 pm, calculate the ionic radius of the halide in pattern C. (4 marks) (ii) In terms of Element X, assign chemical formulae to XRD patterns A to D.
Step by Step Solution
★★★★★
3.45 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


