Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The rate constant for the reaction of a particular organic compound with water varies with temperature as follows: From these data, plot a graph
1. The rate constant for the reaction of a particular organic compound with water varies with temperature as follows:
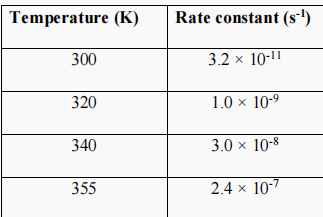
From these data, plot a graph and calculate the activation energy in units of kJ/mol.
2. Differentiate between kinetic and thermodynamic control reactions. Explain the role of temperature in determining the product ratio of the kinetic and thermodynamic products in addition of HCI to 1,3-butadiene.
\begin{tabular}{|c|c|} \hline Temperature (K) & Rate constant (s1) \\ \hline 300 & 3.21011 \\ \hline 320 & 1.0109 \\ \hline 340 & 3.0108 \\ \hline 355 & 2.4107 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


