Question
1. The rate of the homogeneous gas-phase reaction: A + 3B 2X + Y has been studied. Some of the data are: Initial total
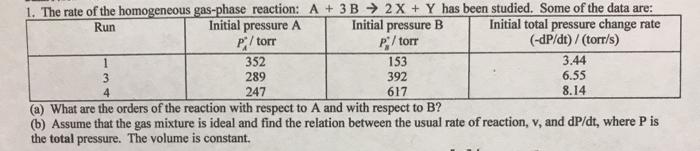
1. The rate of the homogeneous gas-phase reaction: A + 3B 2X + Y has been studied. Some of the data are: Initial total pressure change rate (-dP/dt) / (torr/s) Initial pressure A P/ torr 352 Initial pressure B P/ torr Run 289 247 153 392 3.44 6.55 8.14 3 4 617 (a) What are the orders of the reaction with respect to A and with respect to B? (b) Assume that the gas mixture is ideal and find the relation between the usual rate of reaction, v, and dP/dt, where P is the total pressure. The volume is constant.
Step by Step Solution
3.31 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry Concepts and Connections
Authors: Dean R. Appling, Spencer J. Anthony Cahill, Christopher K. Mathews
1st edition
321839927, 978-0133853490, 133853497, 978-0321839923
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



