Answered step by step
Verified Expert Solution
Question
1 Approved Answer
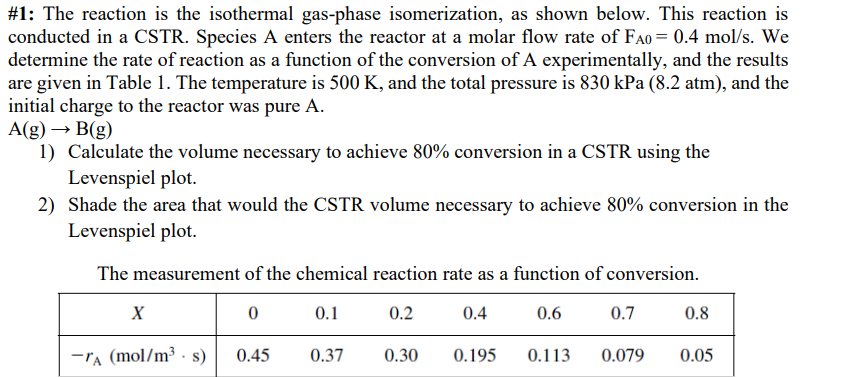
# 1 : The reaction is the isothermal gas - phase isomerization, as shown below. This reaction is conducted in a CSTR . Species A
#: The reaction is the isothermal gasphase isomerization, as shown below. This reaction is
conducted in a CSTR Species A enters the reactor at a molar flow rate of We
determine the rate of reaction as a function of the conversion of A experimentally, and the results
are given in Table The temperature is and the total pressure is kPaatm and the
initial charge to the reactor was pure
Calculate the volume necessary to achieve conversion in a CSTR using the
Levenspiel plot.
Shade the area that would the CSTR volume necessary to achieve conversion in the
Levenspiel plot.
The measurement of the chemical reaction rate as a function of conversion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


