Answered step by step
Verified Expert Solution
Question
1 Approved Answer
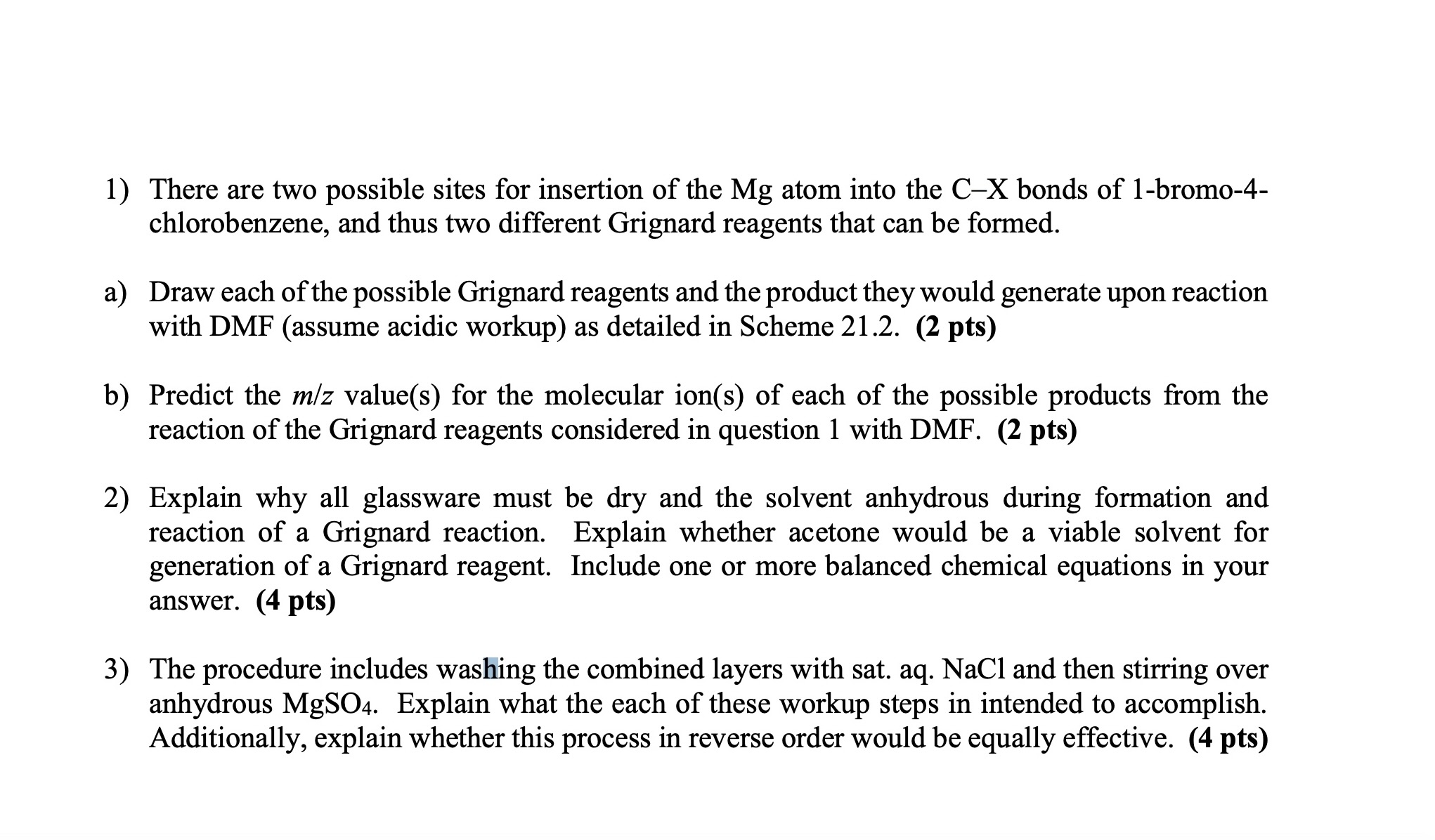
1 ) There are two possible sites for insertion of the Mg atom into the C X bonds of 1 - bromo - 4 -
There are two possible sites for insertion of the Mg atom into the CX bonds of bromo
chlorobenzene, and thus two different Grignard reagents that can be formed.
a Draw each of the possible Grignard reagents and the product they would generate upon reaction
with DMF assume acidic workup as detailed in Scheme pts
b Predict the mz values for the molecular ions of each of the possible products from the
reaction of the Grignard reagents considered in question with DMF pts
Explain why all glassware must be dry and the solvent anhydrous during formation and
reaction of a Grignard reaction. Explain whether acetone would be a viable solvent for
generation of a Grignard reagent. Include one or more balanced chemical equations in your
answer. There are two possible sites for insertion of the atom into the bonds of bromo
chlorobenzene, and thus two different Grignard reagents that can be formed.
a Draw each of the possible Grignard reagents and the product they would generate upon reaction
with DMF assume acidic workup as detailed in Scheme pts
b Predict the values for the molecular ions of each of the possible products from the
reaction of the Grignard reagents considered in question with DMF pts
Explain why all glassware must be dry and the solvent anhydrous during formation and
reaction of a Grignard reaction. Explain whether acetone would be a viable solvent for
generation of a Grignard reagent. Include one or more balanced chemical equations in your
answer. pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


