Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Use Equation 12 to calculate the volume of copper stock solution required to create a copper standard solution with the concentration assigned by

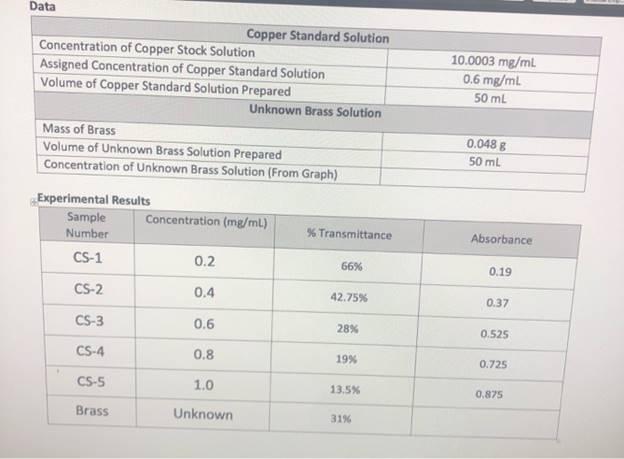
1. Use Equation 12 to calculate the volume of copper stock solution required to create a copper standard solution with the concentration assigned by your instructor (See data table above). 2. Calculate the mass of copper in the unknown brass solution by multiplying the concentration of copper in the unknown brass solution (mg/mL) by the volume of brass solution prepared (mL). Mass of Cu = (Conc. of Cu) x (Volume of Brass Solution) 3. Calculate the percent weight of copper in the unknown brass solution. %wt Cu =Mass of Copper/Mass of Brass x 100 Discussion State the results of the experiment. Which aspects of the experiment likely impacted your ability to determine an accurate percent weight of copper in the brass sample? If you elected to exclude the results from any of the standards when constructing your calibration curve, please explain your rationale. Data Copper Standard Solution Concentration of Copper Stock Solution Assigned Concentration of Copper Standard Solution Volume of Copper Standard Solution Prepared 10.0003 mg/ml 0.6 mg/mL 50 mL Unknown Brass Solution Mass of Brass Volume of Unknown Brass Solution Prepared Concentration of Unknown Brass Solution (From Graph) 0.048 8 50 ml Experimental Results Sample Number Concentration (mg/mL) Transmittance Absorbance CS-1 0.2 66% 0.19 CS-2 0.4 42.75% 0.37 CS-3 0.6 28% 0.525 CS-4 0.8 19% 0.725 CS-5 1.0 13.5% 0.875 Brass Unknown 31%
Step by Step Solution
★★★★★
3.50 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
Explanation Q1 Using equation M1V1stock M2V2standard 100003 Vstock ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


