Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) Use the solubility table and classify each compound as water soluble or water insoluble. Na2CO3Ag2SFeCl3Pb(ClO3)2 Solubility Table Water-Soluble Compounds Insoluble Exception Compounds containing an
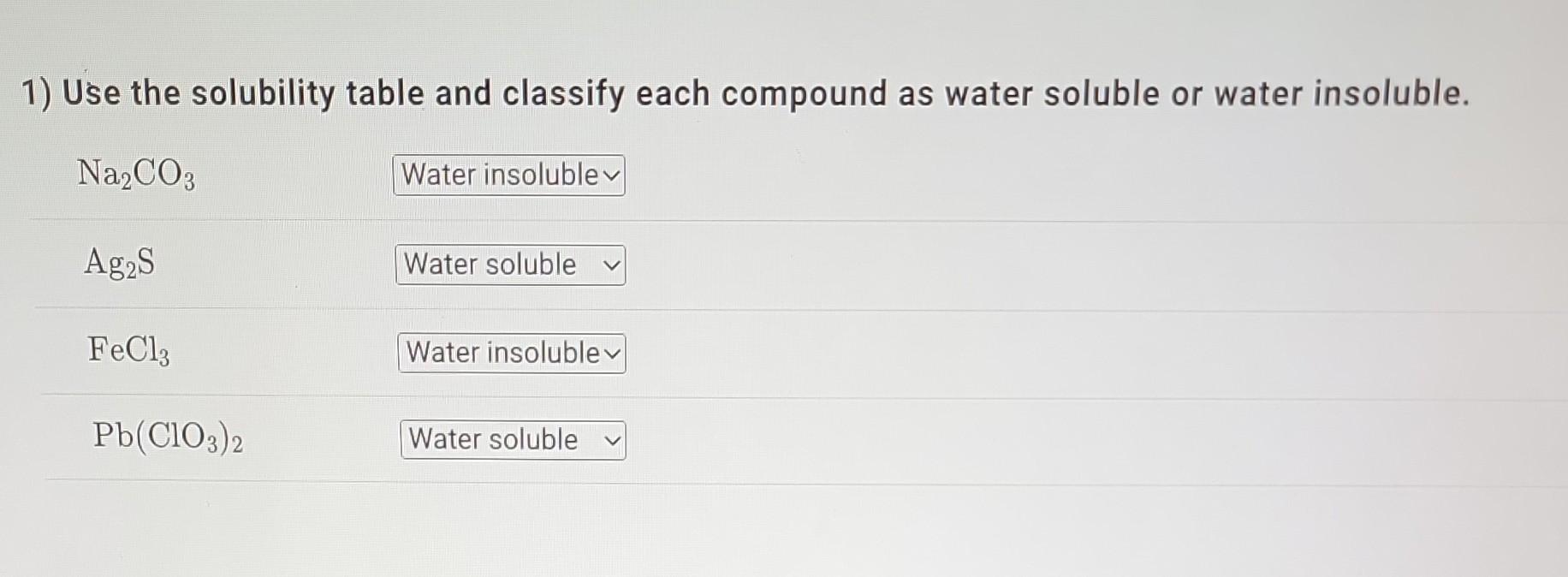
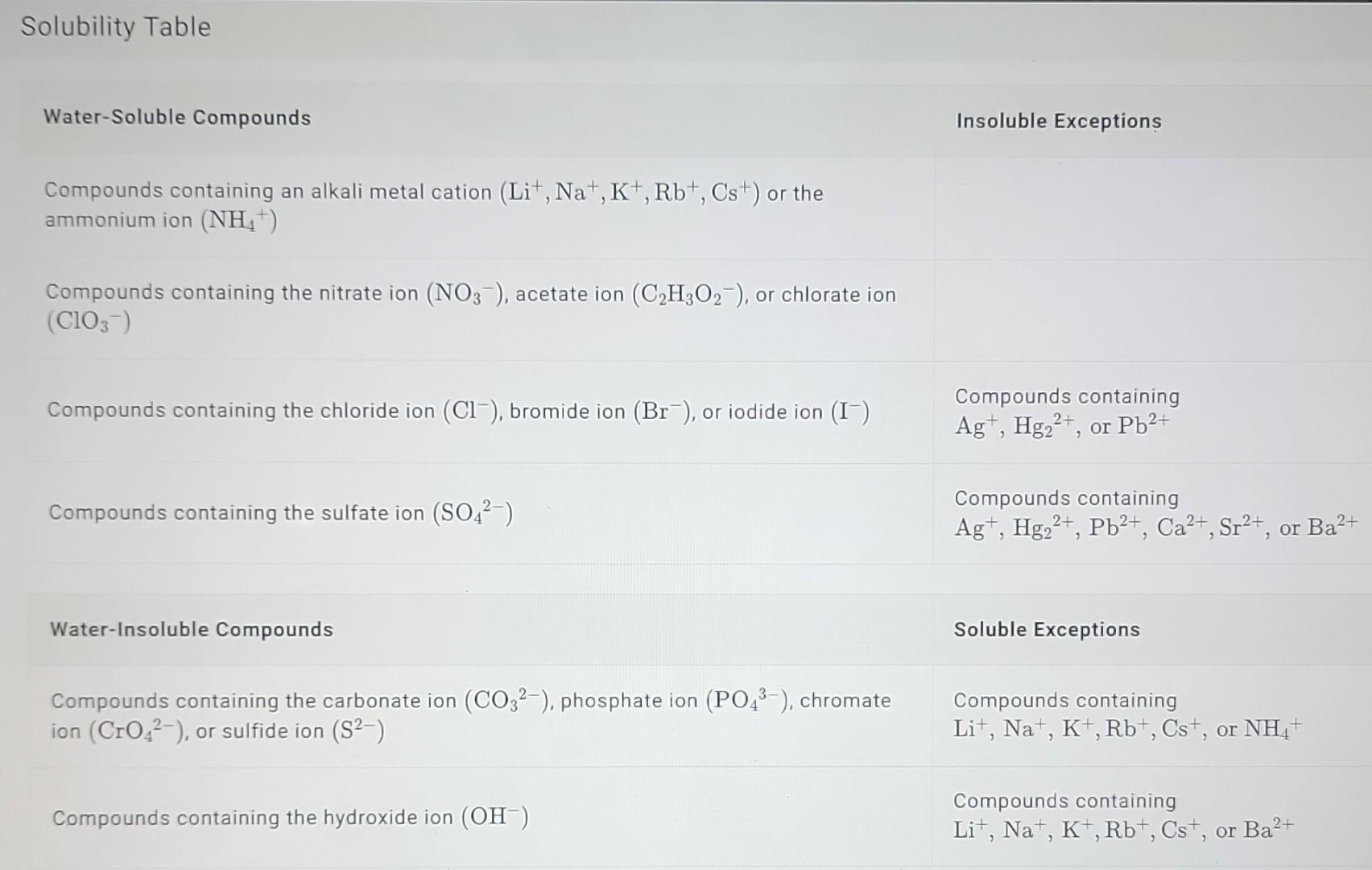
1) Use the solubility table and classify each compound as water soluble or water insoluble. Na2CO3Ag2SFeCl3Pb(ClO3)2 Solubility Table Water-Soluble Compounds Insoluble Exception Compounds containing an alkali metal cation (Li+,Na+,K+,Rb+,Cs+)or the ammonium ion (NH4+) Compounds containing the nitrate ion (NO3), acetate ion (C2H3O2), or chlorate ion (ClO3) Compounds containing the chloride ion (Cl), bromide ion (Br), or iodide ion (I)CompoundscontainingAg+,Hg22+,or2Pb2+ Compounds containing the sulfate ion (SO42) Compounds containing Ag+,Hg22+,Pb2+,Ca2+,Sr2+, or Ba2+ Water-Insoluble Compounds Soluble Exceptions Compounds containing the carbonate ion (CO32), phosphate ion (PO43), chromate Compounds containing ion (CrO42), or sulfide ion (S2)Li+,Na+,K+,Rb+,Cs+,or4NH4+ Compounds containing the hydroxide ion (OH) Compounds containing Li+,Na+,K+,Rb+,Cs+, or Ba2+
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


