Question
1. Using Figure 3.1, determine a. the number of grams of CuSO4, 5H2O that will dissolve in 100g of H2O at 100 Celcius. b. the
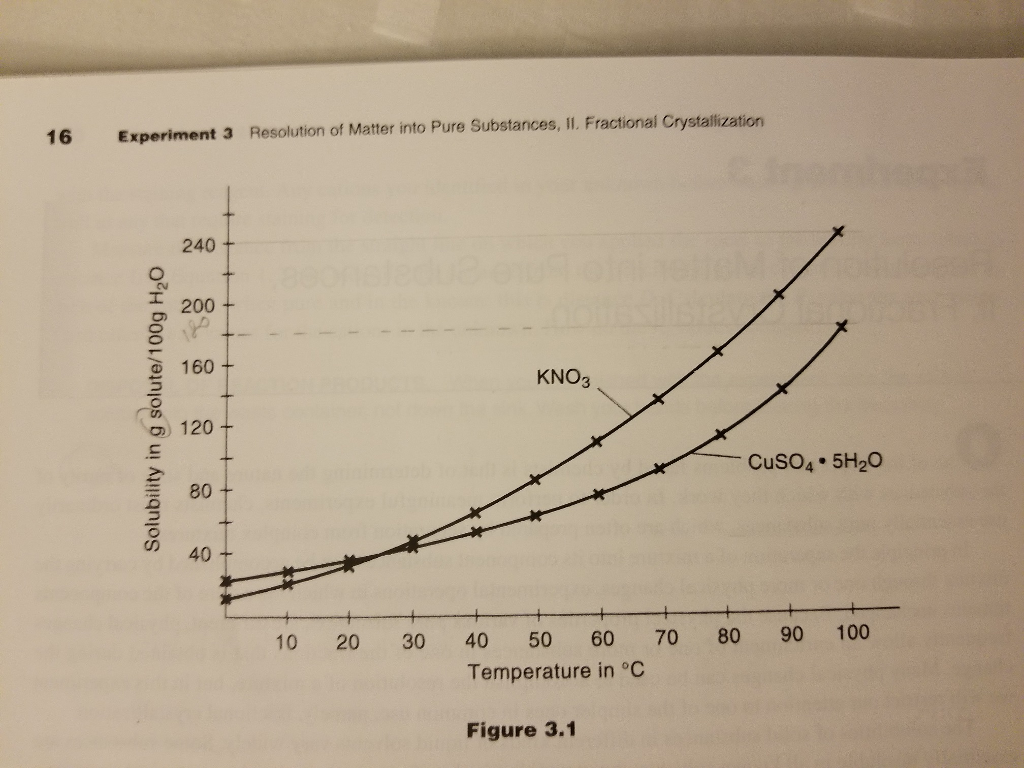
1. Using Figure 3.1, determine
a. the number of grams of CuSO4, 5H2O that will dissolve in 100g of H2O at 100 Celcius.
b. the number of grams of water required to dissolve 4.0 g of CuSO4, 5 H2O at 100 celcius.?
c. the number of grams of water required to dissolve 18 g KNO3 at 100 celcius
d. the number of grams of water required to dissolve a mixture containing 18 g KNO3 and 4.0 g CuSO4, 5 H2O, assuming that the solubility of one substance is not affected by the presence of another
2. To the solution in Problem 1(d) at 100 celcius, an additional 12.0 g of water are added, and the solution is cooled to 0 celcius
a. How much KNO3 remains in solution?
b. How much KNO3 crystallizes out?
c. How much CuSO4, 5 H2O crystallizes out?
d. What percent of the KNO3 in the sample is recovered?
16 Experiment 3 Resolution of Matter into Pure Substances, II. Fractional Crystallization Solubility in g solute/100g HO 240 200 160 120 80 40 10 20 30 40 KNO3 50 Temperature in C 60 70 Figure 3.1 CuSO4.5HO 100 90 80
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


