Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Water is added at a flow rate of 0.2m3/h in order to dilute the salt solution at a concentration of 1mol/m3 and flow rate
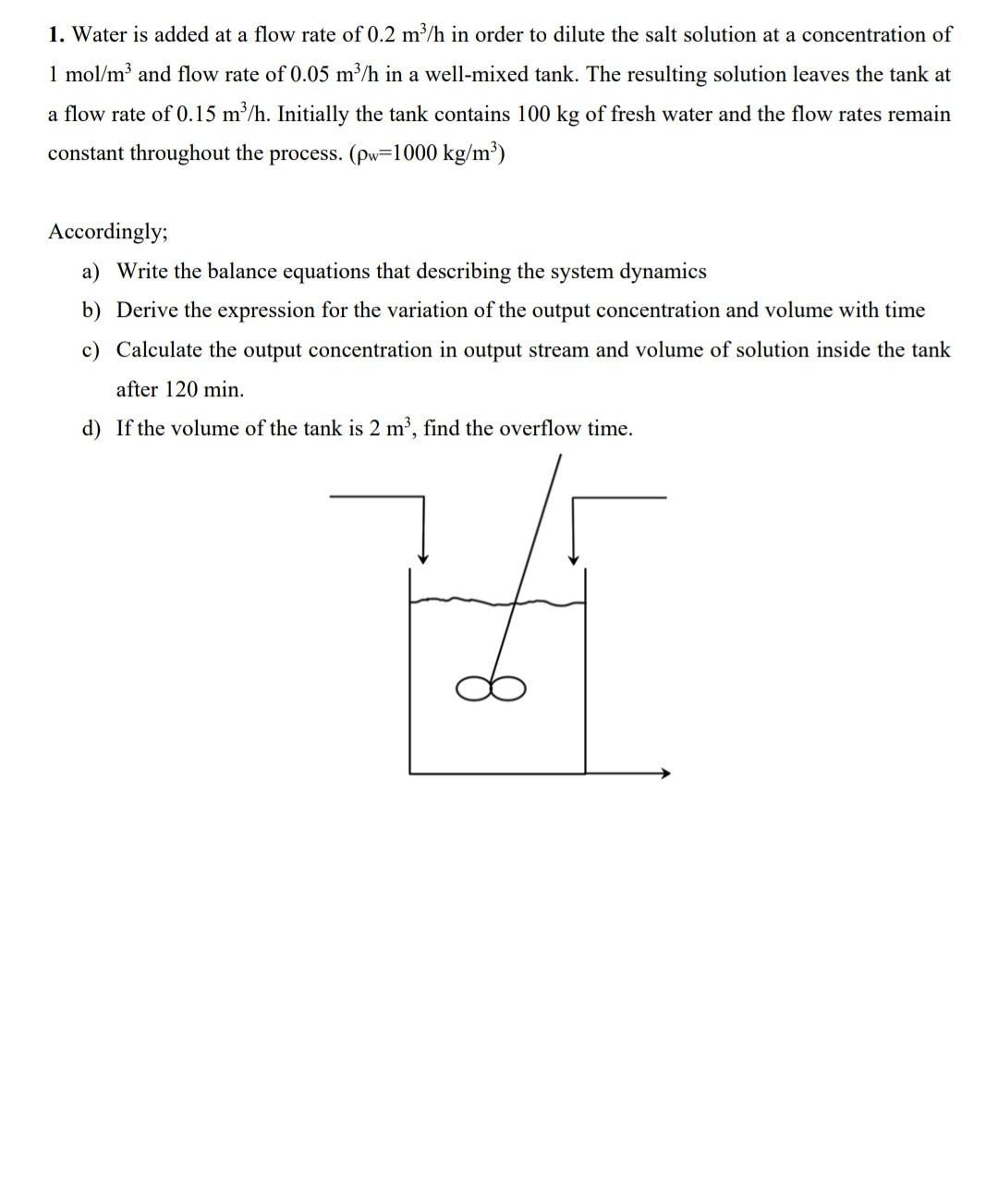
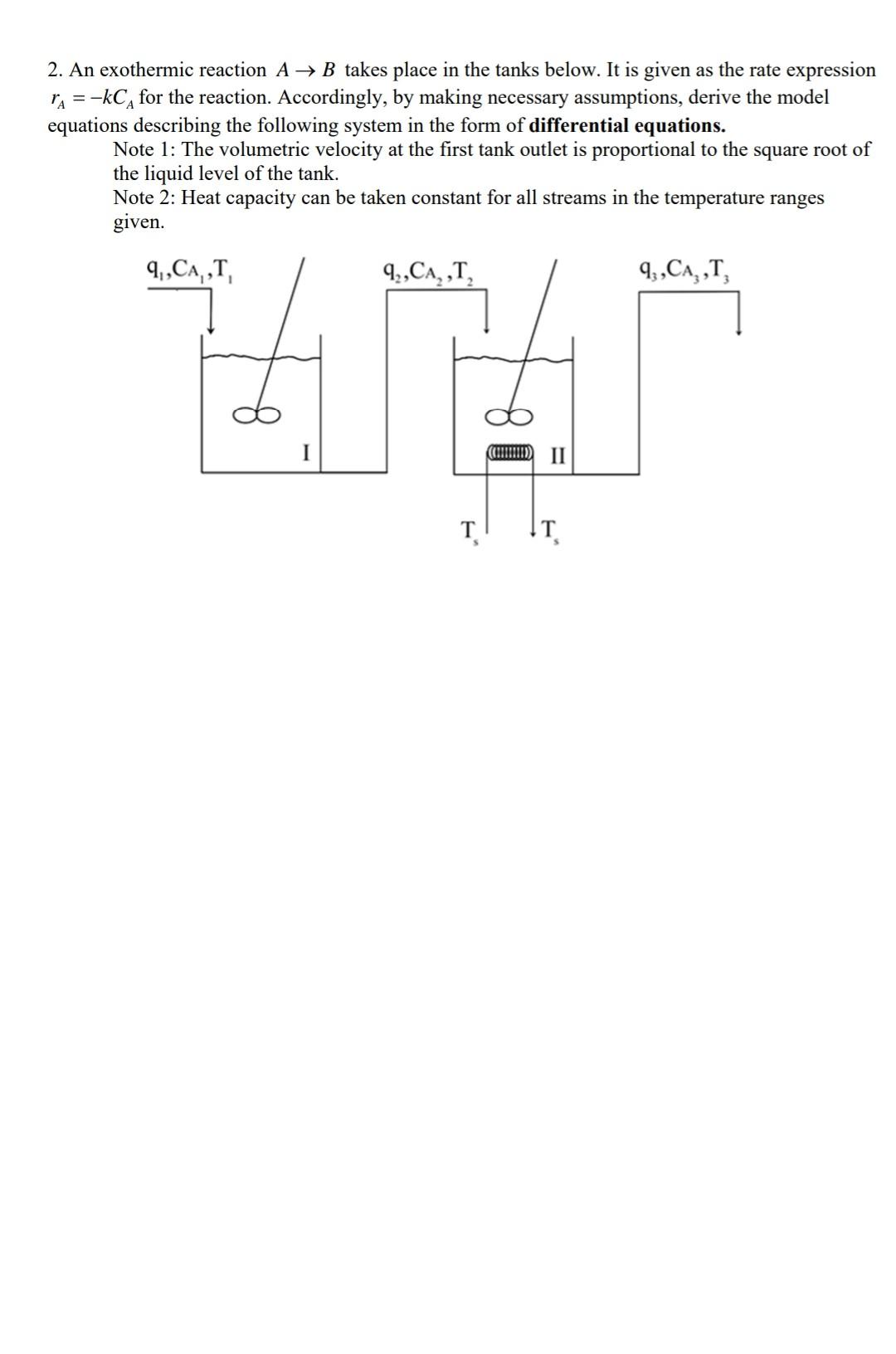

1. Water is added at a flow rate of 0.2m3/h in order to dilute the salt solution at a concentration of 1mol/m3 and flow rate of 0.05m3/h in a well-mixed tank. The resulting solution leaves the tank at a flow rate of 0.15m3/h. Initially the tank contains 100kg of fresh water and the flow rates remain constant throughout the process. (w=1000kg/m3 ) Accordingly; a) Write the balance equations that describing the system dynamics b) Derive the expression for the variation of the output concentration and volume with time c) Calculate the output concentration in output stream and volume of solution inside the tank after 120min. d) If the volume of the tank is 2m3, find the overflow time. 2. An exothermic reaction AB takes place in the tanks below. It is given as the rate expression rA=kCA for the reaction. Accordingly, by making necessary assumptions, derive the model equations describing the following system in the form of differential equations. Note 1: The volumetric velocity at the first tank outlet is proportional to the square root of the liquid level of the tank. Note 2: Heat capacity can be taken constant for all streams in the temperature ranges given. 3. There is a semi-batch reactor with 100LX solution at a concentration of 2mol/L. Inert solution (I) at a concentration of 0.5mol/L is sent to this reactor at a rate of 0.75L/min. Reaction takes place in isothermal conditions in this reactor. Since the rate constant (k) of the reaction is 0.018L/mol.min; XY a) What will be the concentrations of the components (X, Y, I) in the reactor after 30min ? b) What is the time at the moment when the conversion is 0.5 . Note: You can assume that the density of the inert solution and X solution in the tank constant and equal
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


