Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) When accelerating an electron to 50 kV, calculate the energy, momentum, and wavelength of the electron, respectively. (The electron charge is 1.6x10-19C, the electron
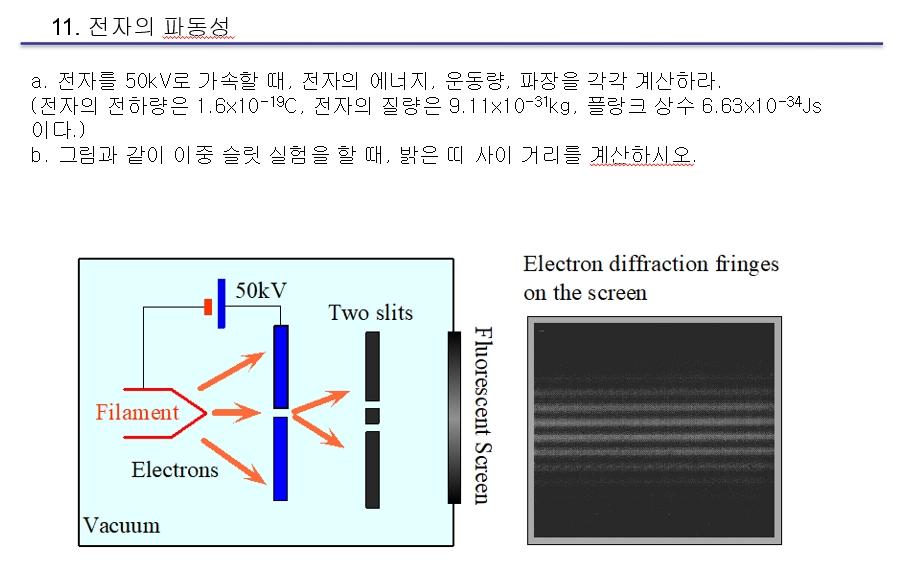
1) When accelerating an electron to 50 kV, calculate the energy, momentum, and wavelength of the electron, respectively. (The electron charge is 1.6x10-19C, the electron mass is 9.11x10-31kg, and the Planck constant is 6.63x10-34Js.)
2) When conducting a double-slit experiment as shown, calculate the distance between the bright bands.
11. a. 50kV , , , . ( 1.610-19, 9.1110-31kg, 6.6310-34 Js .) b. , . Electron diffraction fringes on the screen 50kV Two slits Filament Fluorescent Screen Electrons VacuumStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


