Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Write the mass balances including the extent of reaction for the following process: 2. What is the degree of freedom? 3. Minimum amount
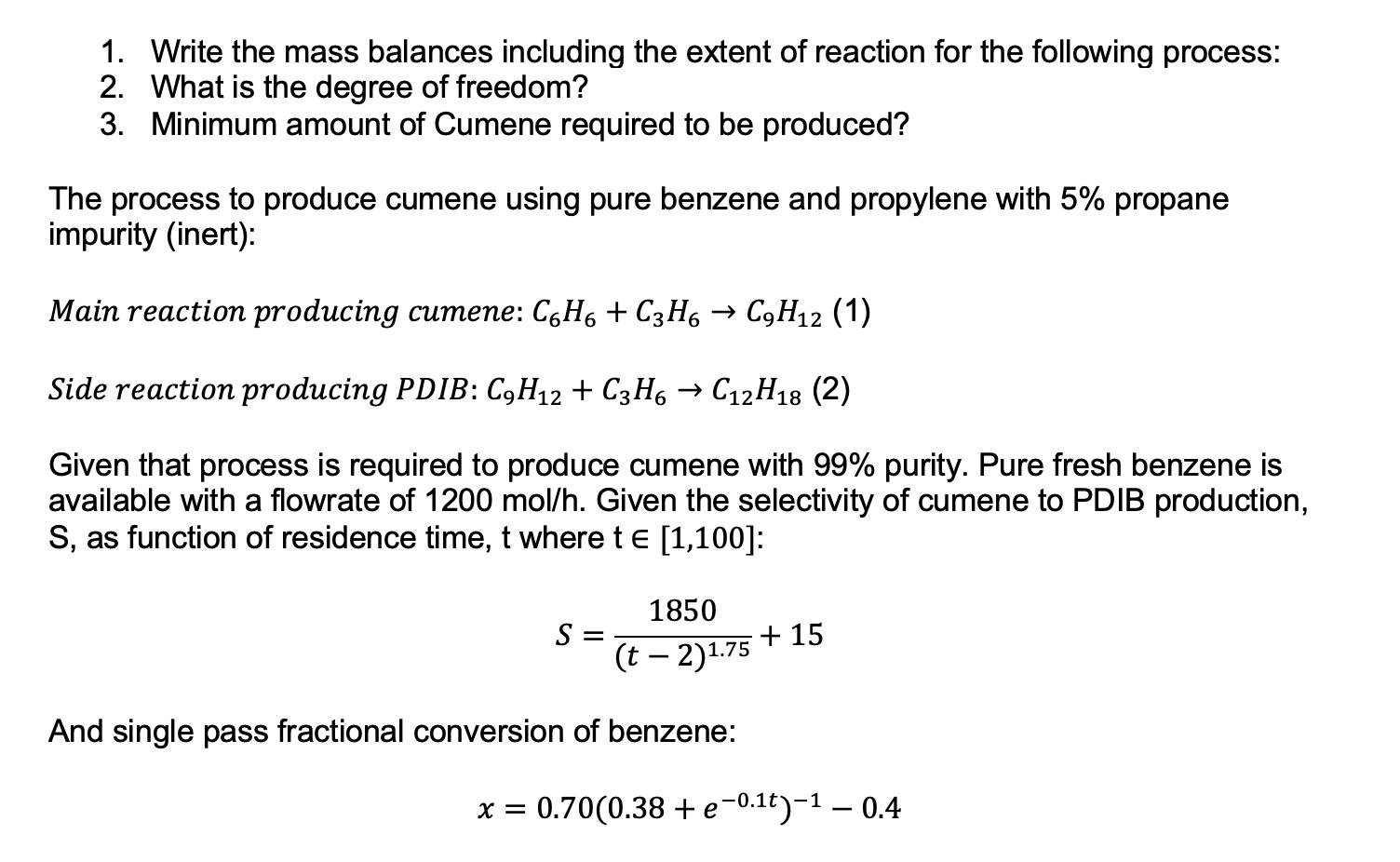
1. Write the mass balances including the extent of reaction for the following process: 2. What is the degree of freedom? 3. Minimum amount of Cumene required to be produced? The process to produce cumene using pure benzene and propylene with 5% propane impurity (inert): Main reaction producing cumene: C6H6 + C3H6 C9H2 (1) Side reaction producing PDIB: C9H12 + C3H6 C12H18 (2) Given that process is required to produce cumene with 99% purity. Pure fresh benzene is available with a flowrate of 1200 mol/h. Given the selectivity of cumene to PDIB production, S, as function of residence time, t where t = [1,100]: 1850 (t - 2)1.75 And single pass fractional conversion of benzene: S = + 15 x = 0.70(0.38 + e-0.t)- -0.4
Step by Step Solution
★★★★★
3.53 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
1 Mass Balances with Extent of Reaction We can set up mass balances for each component involved in the reactions Lets denote F Molar flowrate molh x F...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


