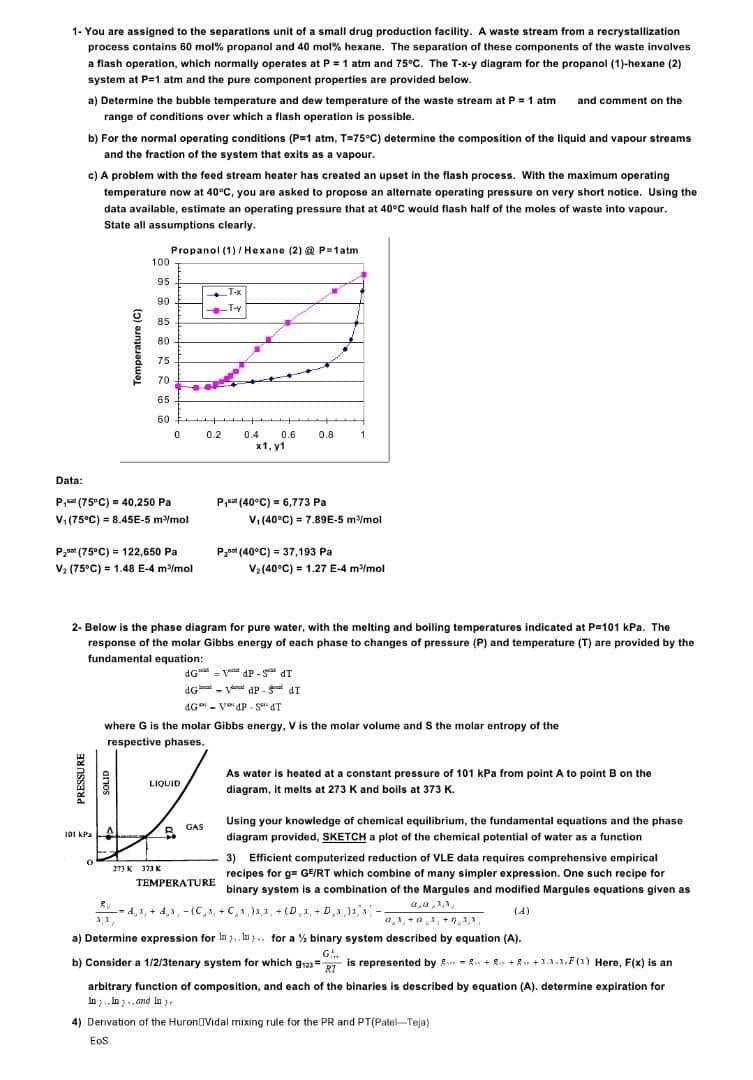
1. You are assigned to the separations unit of a small drug production facility. A waste stream from a recrystallization process contains 60 mol% propanol and 40 mol% hexane. The separation of these components of the waste involves a flash operation, which normally operates at P = 1 atm and 75C. The T-x-y diagram for the propanol (1)-hexane (2) system at P=1 atm and the pure component properties are provided below. a) Determine the bubble temperature and dew temperature of the waste stream at P = 1 atm and comment on the range of conditions over which a flash operation is possible. b) For the normal operating conditions (P=1 atm, T-75C) determine the composition of the liquid and vapour streams and the fraction of the system that exits as a vapour. c) A problem with the feed stream heater has created an upset in the flash process. With the maximum operating temperature now at 40C, you are asked to propose an alternate operating pressure on very short notice. Using the data available, estimate an operating pressure that at 40C would flash half of the moles of waste into vapour. State all assumptions clearly. Propanol (1) / Hexane (2) @ potatm 100 95 90 T-X -TY 85 80 Temperature (C) 75 70 65 60 0 0.2 0.8 1 0.4 0.6 x1, y1 Data: P,- (75C) = 40,250 Pa V. (75C) = 8.45E-5 m-/mol = - P (40C) = 6,773 Pa V. (40C) = 7.89E-5 mmol P200 (75C) = 122,650 Pa V2 (75C) = 1.48 E-4 m-/mol ) = E-4 P_0=t(40C) = 37,193 Pa (= Vz(40C) = 1.27 E-4 m/mol = 4 2- Below is the phase diagram for pure water, with the melting and boiling temperatures indicated at P=101 kPa. The response of the molar Gibbs energy of each phase to changes of pressure (P) and temperature (T) are provided by the fundamental equation: dGdP- ST dGV aP - 5- IT dG-VP-SAT 4G where G is the molar Gibbs energy, V is the molar volume and the molar entropy of the respective phases. PRESSURE LIQUID As water is hosted at a constant pressure of 101 kPa from point A to point B on the diagram, it melts at 273 K and boils at 373 K. . It GAS A 0 Using your knowledge of chemical equilibrium, the fundamental equations and the phase 101 kPa diagram provided, SKETCH a plot of the chemical potential of water as a function 3) Efficient computerized reduction of VLE data requires comprehensive empirical 27 K 373 recipes for g-GERT which combine of many simpler expression. One such recipe for TEMPERATURE binary system is a combination of the Margules and modified Margules equations given as Bu - 4,4+2,2-0,4+0,1,2,1, +(D 1,+D,2,1'* 0.0.1 ,{ (4) a) Determine expression for In In for a % binary system described by equation (A). G b) Consider a 1/2/3tenary system for which gta = 2.7 is represented by Ron = +*+&+1.1.FC) Here, F(X) is an arbitrary function of composition, and each of the binaries is described by equation (A). determine expiration for In In .. and in 4) Derivation of the HuronVidal mixing rule for the PR and PT(Patel-Teja) Eos a







