Question
1. Your task is to produce 510 mg of H-TPP. Calculate the following. Report all answers to 3 sf a. the mL of pyrrole required
1. Your task is to produce 510 mg of H-TPP. Calculate the following. Report all answers to 3 sf
a. the mL of pyrrole required
b. the mL of aldehyde required
c. the mL of propionic acid required
d. the theoretical yield of your reaction in grams
2. A Chem332L student found a second journal article in which the authors used an improved method which increased the percent yield of the reaction to 33.7%.
Following the procedure reported in this second article, the student designed an experiment to isolate 0.337 mg of H-TPP. He snuck into lab (wearing PPE and a face mask), ran the experiment, and obtained 0.13 mg of beautiful purple crystals. What was the percent yield of his reaction?
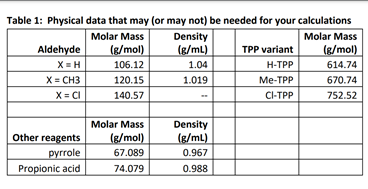
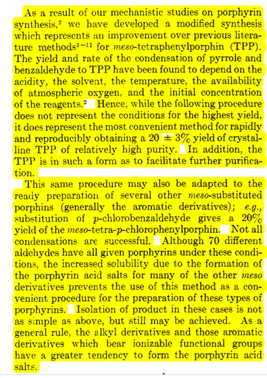
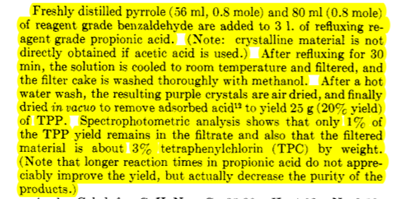
Table 1: Physical data that may (or may not) be needed for your calculations Molar Mass Density Molar Mass Aldehyde (g/mol) (g/mL) TPP variant (g/mol) X=H 106.12 1.04 H-TPP 614.74 X = CH3 120.15 1.019 Me-TPP 670.74 X = CI 140.57 CI-TPP 752.52 Other reagents pyrrole Propionic acid Molar Mass (g/mol) 67.089 74.079 Density (g/mL) 0.967 0.988 As a result of our mechanistic studies on porphyrin synthesis, we have developed a modified synthesis which represents an improvement over previous litera- ture methods for meso-tetraphenylporphin (TPP). The yield and rate of the condensation of pyrrole and benzaldehyde to TPP have been found to depend on the acidity, the solvent, the temperature, the availability of atmospheric oxygen, and the initial concentration of the reagents. Hence, while the following procedure does not represent the conditions for the highest yield, it does represent the most convenient method for rapidly and reproducibly obtaining a 20 + 3% yield of crystal- line TPP of relatively high purity. In addition, the TPP is in such a form as to facilitate further purifica- tion. This same procedure may also be adapted to the really preparation of several other meso-substituted porphins (generally the aromatic derivatives); e.g. substitution of p-chlorobenzaldehyde gives a 20% yield of the meso-tetra-p-chlorophenylporphin. Not all condensations are successful. Although 70 different aldehydes have all given porphyrins under these condi- tions, the increased solubility due to the formation of the porphyrin acid salts for many of the other meso derivatives prevents the use of this method as a con- venient procedure for the preparation of these types of porphyrins. Isolation of product in these cases is not as simple as above, but still may be achieved. As a general rule, the alkyl derivatives and those aromatie derivatives which bear ionizable functional groups have a greater tendency to form the porphyrin acid salts. Freshly distilled pyrrole (56 ml, 0.8 mole) and 80 ml (0.8 mole) of reagent grade benzaldehyde are added to 3 1. of refluxing re- agent grade propionic acid. (Note: crystalline material is not directly obtained if acetic acid is used.) After refluxing for 30 min, the solution is cooled to room temperature and filtered, and the filter cake is washed thoroughly with methanol. After a hot water wash, the resulting purple crystals are air dried, and finally dried in vacuo to remove adsorbed acid to yield 25 g (20% yield) of TPP. Spectrophotometric analysis shows that only 1% of the TPP yield remains in the filtrate and also that the filtered material is about 3% tetraphenylchlorin (TPC) by weight. (Note that longer reaction times in propionic acid do not appre- ciably improve the yield, but actually decrease the purity of the products.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


