Question: 10. [-/4 Points] SERPSE10 21.A.0P.040. ASK YOUR TEACHER PRACTICE ANOTHER A cold room used for low-temperature research is maintained at a constant temperature of 7.00C.
![10. [-/4 Points] SERPSE10 21.A.0P.040. ASK YOUR TEACHER PRACTICE ANOTHER A](https://s3.amazonaws.com/si.experts.images/answers/2024/06/6676cd99a7c26_9136676cd998e3df.jpg)
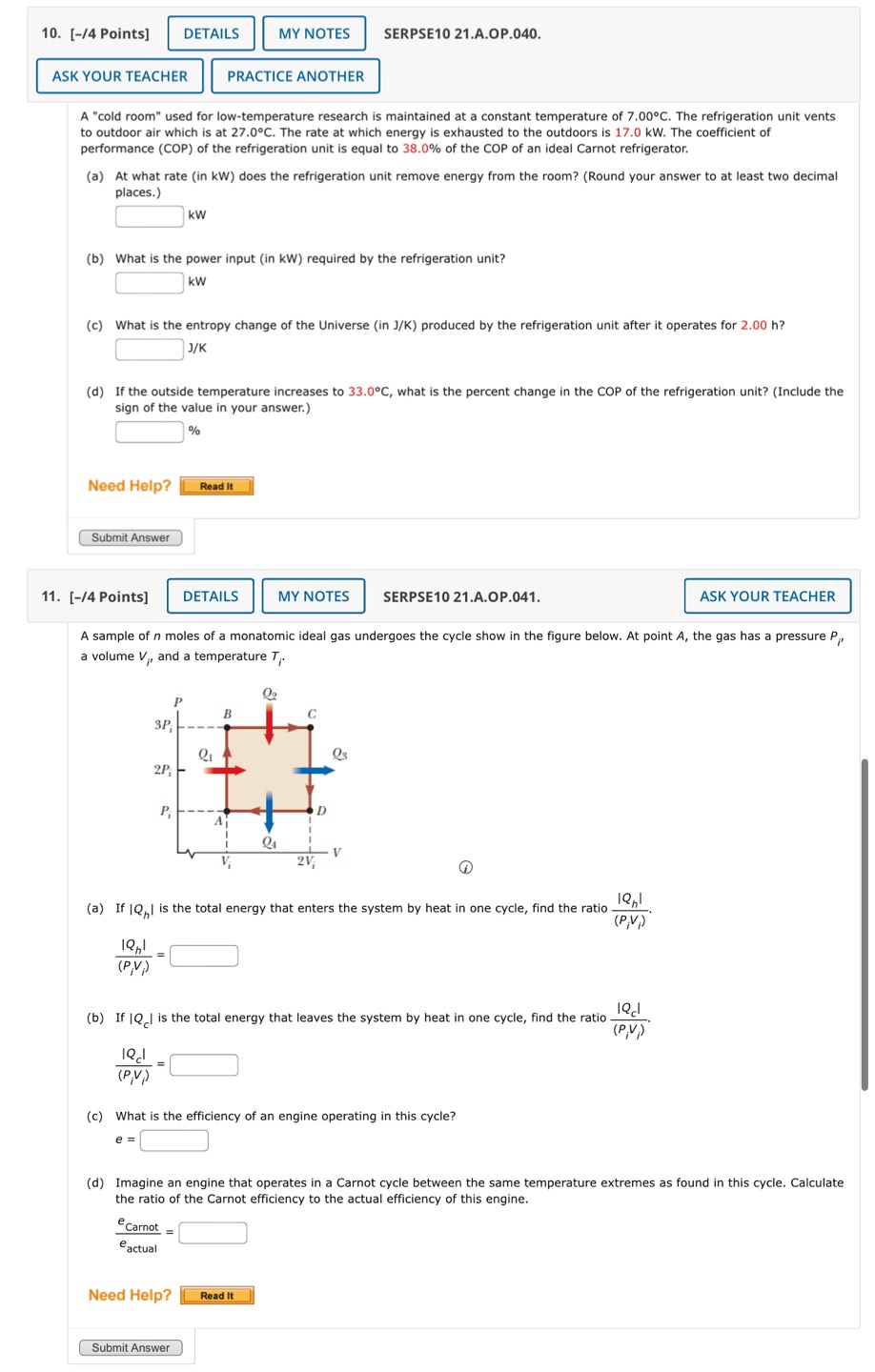
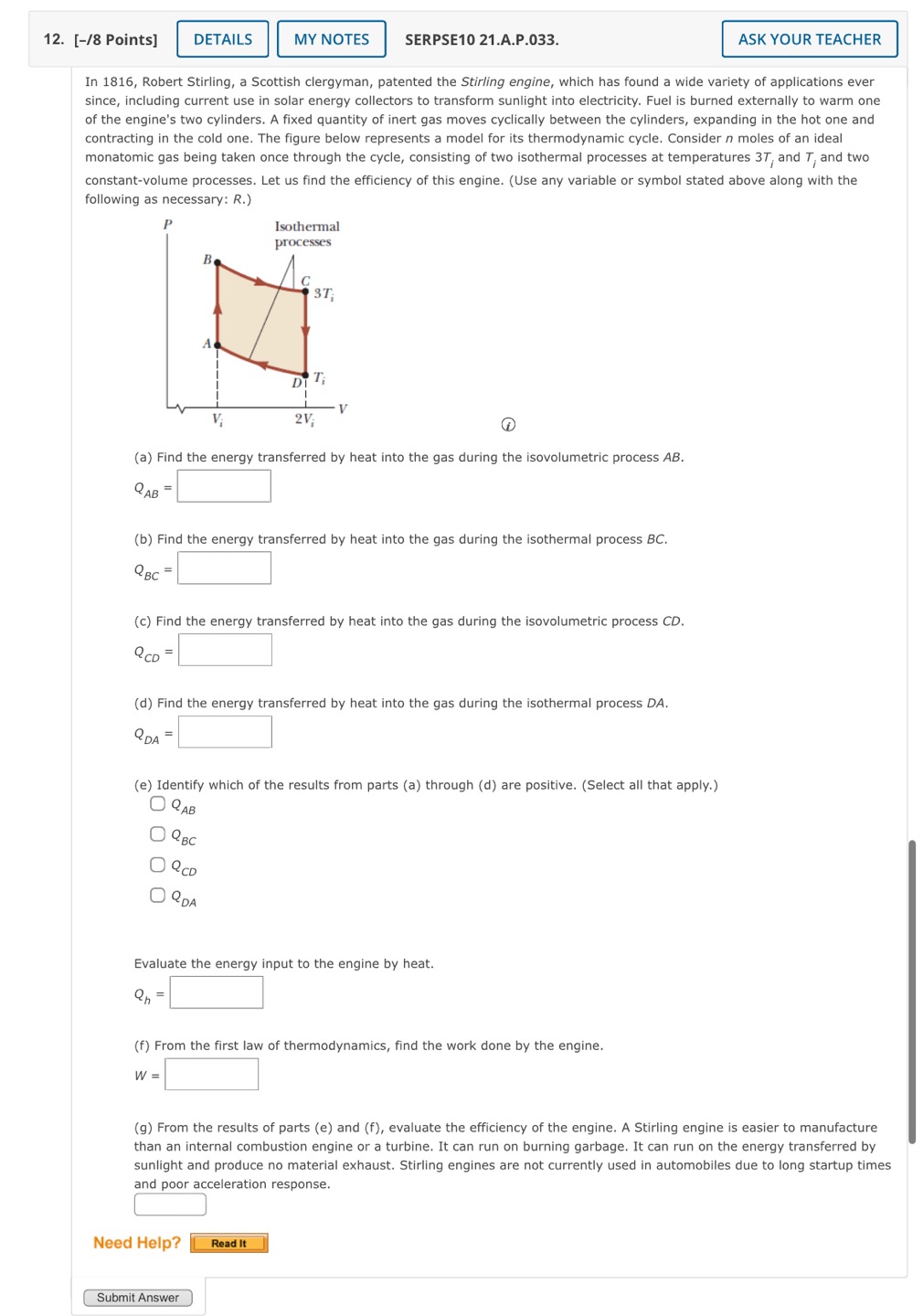
10. [-/4 Points] SERPSE10 21.A.0P.040. ASK YOUR TEACHER PRACTICE ANOTHER A "cold room used for low-temperature research is maintained at a constant temperature of 7.00C. The refrigeration unit vents to outdoor air which is at 27.0C. The rate at which energy is exhausted to the outdoors is 17.0 kW. The coefficient of performance (COP) of the refrigeration unit is equal to 38.0% of the COP of an ideal Carnot refrigerator. (a) At what rate (in kW) does the refrigeration unit remove energy from the room? (Round your answer to at least two decimal places.) YW (b) What is the power input (in kW) required by the refrigeration unit? ! Tkw (c) What is the entropy change of the Universe (in J/K) produced by the refrigeration unit after it operates for 2.00 h? [ /K (d) If the outside temperature increases to 33.0C, what is the percent change in the COP of the refrigeration unit? (Include the sign of the value in your answer.) % Need Help? [[TResan ) Submit Answer 11. [-/4 Points] DETAILS MY NOTES SERPSE10 21.A.OP.041. ASK YOUR TEACHER A sample of n moles of a monatomic ideal gas undergoes the cycle show in the figure below. At point A, the gas has a pressure Py a volume V, and a temperature T @ 1,1 (a) If |Q,| is the total energy that enters the system by heat in one cycle, find the ratio "_, PV 19,1 _ | ry) - J (b) If |Q_| is the total energy that leaves the system by heat in one cycle, find the ratio (Ifl';l). i' 2.l (PV) 2 [ (c) What is the efficiency of an engine operating in this cycle? . ) (d) Imagine an engine that operates in a Carnot cycle between the same temperature extremes as found in this cycle. Calculate the ratio of the Carnot efficiency to the actual efficiency of this engine. Ccamot _ Cactual Need Help? Read It Submit Answer 12. [-/8 Points] DETAILS MY NOTES SERPSE10 21.A.P.033. ASK YOUR TEACHER In 1816, Robert Stirling, a Scottish clergyman, patented the Stirling engine, which has found a wide variety of applications ever since, including current use in solar energy collectors to transform sunlight into electricity. Fuel is burned externally to warm one of the engine's two cylinders. A fixed quantity of inert gas moves cyclically between the cylinders, expanding in the hot one and contracting in the cold one. The figure below represents a model for its thermodynamic cycle. Consider n moles of an ideal monatomic gas being taken once through the cycle, consisting of two isothermal processes at temperatures 37, and T and two constant-volume processes. Let us find the efficiency of this engine. (Use any variable or symbol stated above along with the following as necessary: R.) P Isothermal 'PI'I!CEHES B C ST, A I s | o T | v 2V, @ (a) Find the energy transferred by heat into the gas during the isovolumetric process AB. Qg = ' (b) Find the energy transferred by heat into the gas during the isothermal process BC. Qsc=' (c) Find the energy transferred by heat into the gas during the isovolumetric process CD. 2 (d) Find the energy transferred by heat into the gas during the isothermal process DA. Qg = | (e) Identify which of the results from parts (a) through (d) are positive. (Select all that apply.) D D 9 (@ - Evaluate the energy input to the engine by heat. Q, = (f) From the first law of thermodynamics, find the work done by the engine. (g) From the results of parts (e) and (f), evaluate the efficiency of the engine. A Stirling engine is easier to manufacture than an internal combustion engine or a turbine. It can run on burning garbage. It can run on the energy transferred by sunlight and produce no material exhaust. Stirling engines are not currently used in automobiles due to long startup times and poor acceleration response. W= Need Help? Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


