Question
) (10%) A spherical particle B is surrounded by a gas mixture. The component A diffuses through the gas mixture and reacts with B
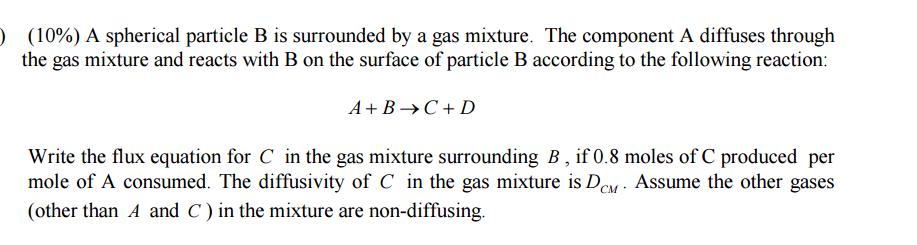
) (10%) A spherical particle B is surrounded by a gas mixture. The component A diffuses through the gas mixture and reacts with B on the surface of particle B according to the following reaction: A+B C + D Write the flux equation for C in the gas mixture surrounding B, if 0.8 moles of C produced per mole of A consumed. The diffusivity of C in the gas mixture is DCM. Assume the other gases (other than A and C) in the mixture are non-diffusing.
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
87 Diffusing hom And 96 IV Ginczn Moles of c 08 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Classical Electrodynamics
Authors: John David Jackson
3rd Edition
047130932X, 978-0471309321
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



