Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.0 grams of a sample containing phosphorus was dissolved into PO, and the sample was diluted to 500 mL with distilled water. 25.0 mL of
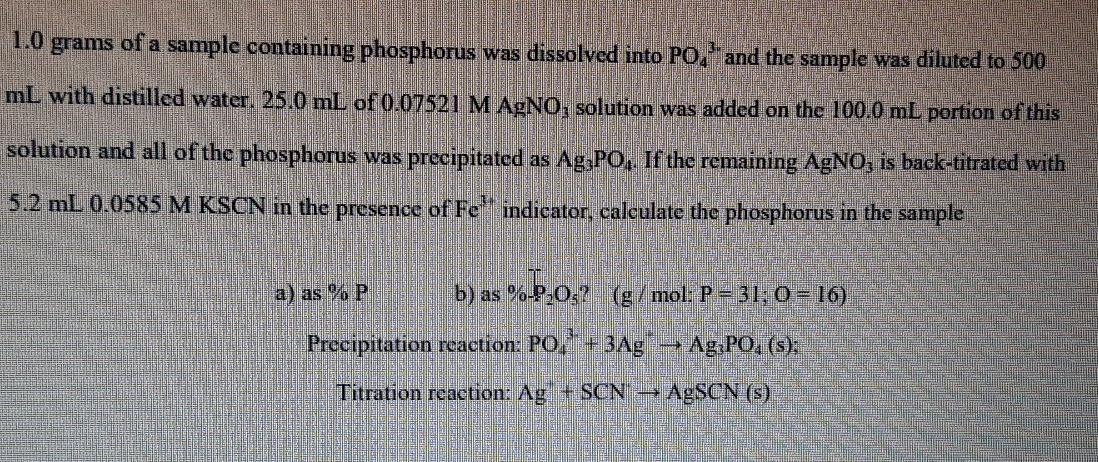
1.0 grams of a sample containing phosphorus was dissolved into PO, and the sample was diluted to 500 mL with distilled water. 25.0 mL of 0.07521 M AgNO, solution was added on the 100.0 mL portion of this solution and all of the phosphorus was precipitated as Ag;PO, If the remaining AgNO, is back-titrated with 5.2 mL 0.0585 M KSCN in the presence of Fe' indicator, calculate the phosphorus in the sample a) as % P b) as %2,0,? ( /mol: P = 31; 0 = 10) Precipitation reaction: P0, + 3Ag Ag3PO, (s): Titration reaction: Ag + SCN --- AgSCN (s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


