Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(10%) Problem 3: A large room contains a volume of air V = 19 m at a temperature of T = 51 F. It
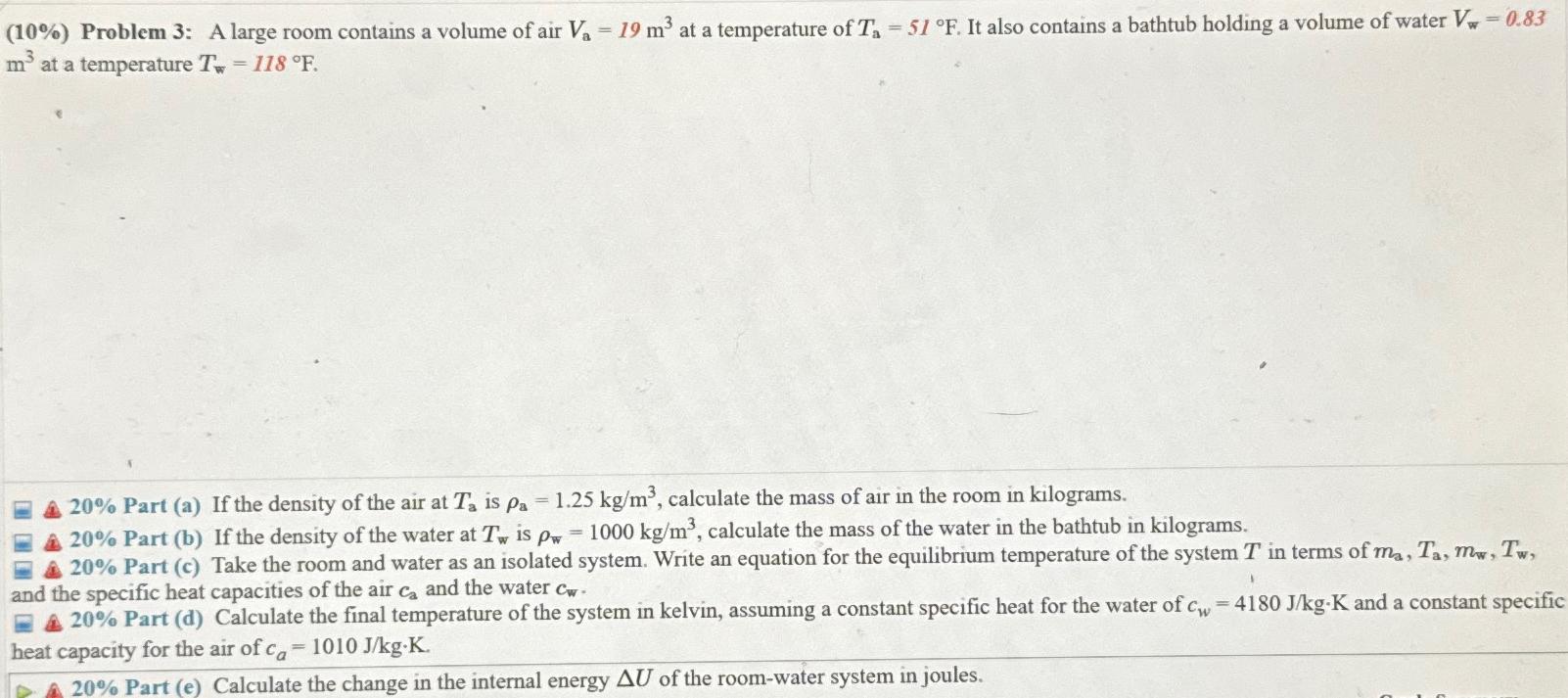
(10%) Problem 3: A large room contains a volume of air V = 19 m at a temperature of T = 51 F. It also contains a bathtub holding a volume of water V, - 0.83 m at a temperature T = 118 F. A 20% Part (a) If the density of the air at Ta is pa = 1.25 kg/m, calculate the mass of air in the room in kilograms. A 20% Part (b) If the density of the water at T is pw = 1000 kg/m, calculate the mass of the water in the bathtub in kilograms. 20% Part (c) Take the room and water as an isolated system. Write an equation for the equilibrium temperature of the system T in terms of ma, Ta, mw, Tw, and the specific heat capacities of the air ca and the water cw- 1 20% Part (d) Calculate the final temperature of the system in kelvin, assuming a constant specific heat for the water of c heat capacity for the air of ca = 1010 J/kg-K. A 20% Part (e) Calculate the change in the internal energy AU of the room-water system in joules. = 4180 J/kg-K and a constant specific
Step by Step Solution
★★★★★
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
a Mass of air in the room mass density volume mass 125 kgm 19 m mass 2375 kg Therefore the mass of air in the room is 2375 kg b Mass of water in the b...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


