Answered step by step
Verified Expert Solution
Question
1 Approved Answer
10 Question 17 The recombination of iodine atoms in the gas phase in the presence of argon was investigated and the order of the reaction
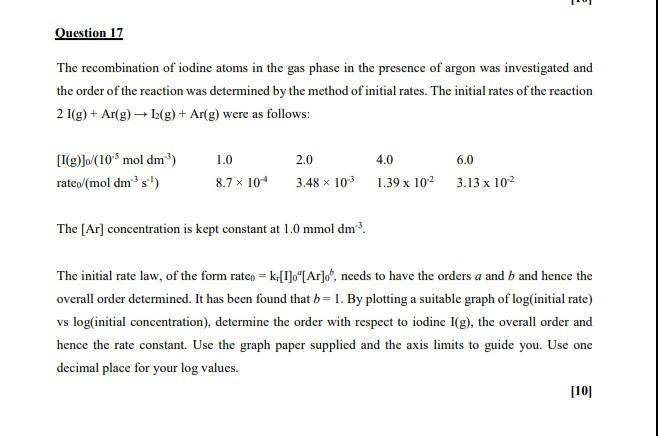
10 Question 17 The recombination of iodine atoms in the gas phase in the presence of argon was investigated and the order of the reaction was determined by the method of initial rates. The initial rates of the reaction 2 1(g) + Ar(g) 12(g) + Arg) were as follows: [1(g)]/(10 mol dm) rateo (mol dm's!) 1.0 8.7 * 10+ 2.0 4.0 6.0 3.48 x 103 1.39 x 102 3.13 x 102 The [Ar] concentration is kept constant at 1.0 mmol dm?. The initial rate law, of the form raten = k.[1]."[Ar]o", needs to have the orders a and b and hence the overall order determined. It has been found that b= 1. By plotting a suitable graph of log(initial rate) vs log(initial concentration), determine the order with respect to iodine I(g), the overall order and hence the rate constant. Use the graph paper supplied and the axis limits to guide you. Use one decimal place for your log values. [10]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


