Answered step by step
Verified Expert Solution
Question
1 Approved Answer
10. The combustion of undecane (156,35 g/mol) proceeds via C, H4 +170, ICO, +12H,0... 43.94 g undecane reacts with 172.76 g oxygen. what is the
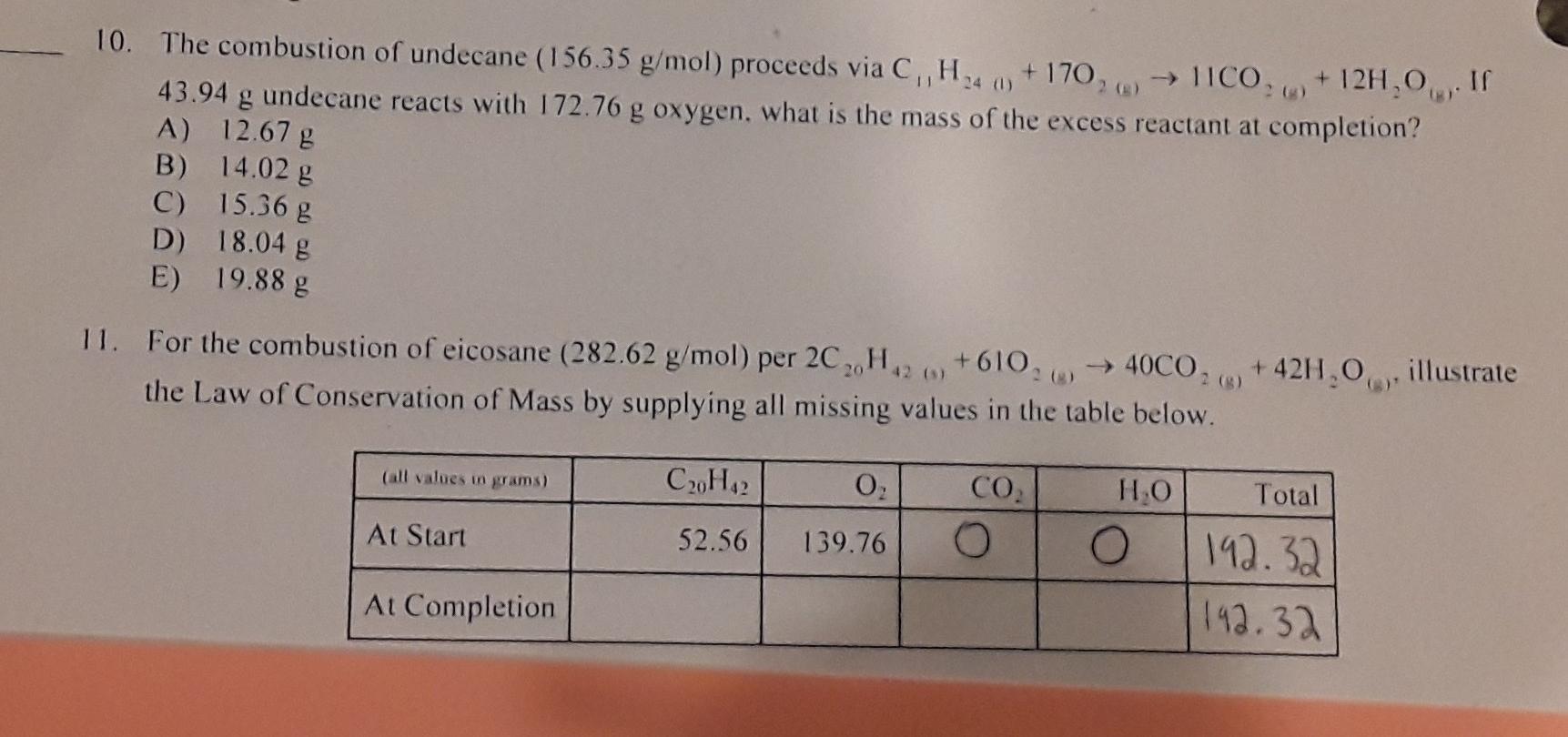
10. The combustion of undecane (156,35 g/mol) proceeds via C, H4 +170, ICO, +12H,0... 43.94 g undecane reacts with 172.76 g oxygen. what is the mass of the excess reactant at completion? A) 12.67 g B) 14.02 g C) 15.36 g D) 18.04 g E) 19.88 g 11. For the combustion of eicosane (282.62 g/mol) per 2CH...+610, 4000, +42H,0illustrate the Law of Conservation of Mass by supplying all missing values in the table below. C20Hz O, CO HO Total At Start 52.56 139.76 O O 192.32 At Completion 192.32 (all values in grams)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


