Answered step by step
Verified Expert Solution
Question
1 Approved Answer
100 mol/hr of an ethanol/HO mixture containing 10 mol% ethanol is to be produced as the liquid outlet stream from an isothermal (T-78.15C) flash
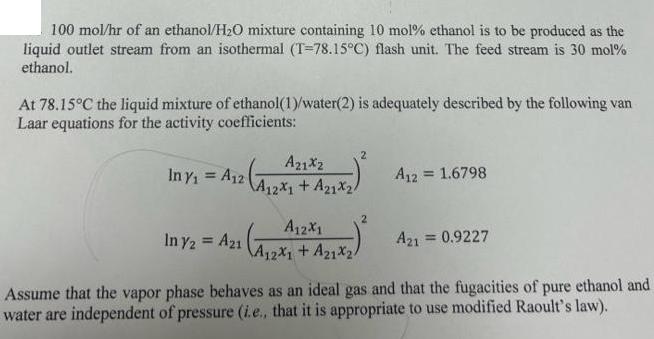

100 mol/hr of an ethanol/HO mixture containing 10 mol% ethanol is to be produced as the liquid outlet stream from an isothermal (T-78.15C) flash unit. The feed stream is 30 mol% ethanol. At 78.15C the liquid mixture of ethanol(1)/water(2) is adequately described by the following van Laar equations for the activity coefficients: Az1Xz Iny = A12 A12x1 + A1X/ A21x24 In y2 = A21 2 A12X1 A12X1 + A21x2/ A12 = 1.6798 A21 = 0.9227 Assume that the vapor phase behaves as an ideal gas and that the fugacities of pure ethanol and water are independent of pressure (i.e., that it is appropriate to use modified Raoult's law). At 78.15C the saturation pressure of pure ethanol is 755 mm Hg and the saturation pressure of pure water is 329 mm Hg. Determine: (i) (ii) the outlet pressure at which the flash must be operated. the composition of the vapor stream. the flow rates of the feed and vapor streams.
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Lets break this down stepbystep Feed stream 100 molhr total 30 mol ethanol 30 mol ethanol 70 mol wat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


