Answered step by step
Verified Expert Solution
Question
1 Approved Answer
100.0mg of basic b(4)II lead phosphate was dissolved in water. Its lead content is precipitated after a suitable reaction in the form of lead chromate.II.
100.0mg of basic b(4)II lead phosphate was dissolved in water. Its lead content is precipitated after a suitable reaction in the form of lead chromate.II. This precipitate in an acidic KI solution forms an amount of iodine that can discolor a solution of sodium thiosulfate with a volume of 11.22 cm3 and a concentration of 0.100mol/dm3 What is the formula of the compoun? 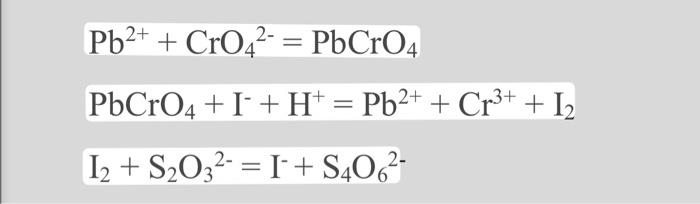
Pb2++CrO42=PbCrO4 PbCrO4+I+H+=Pb2++Cr3++I2 I2+S2O32=I+S4O62 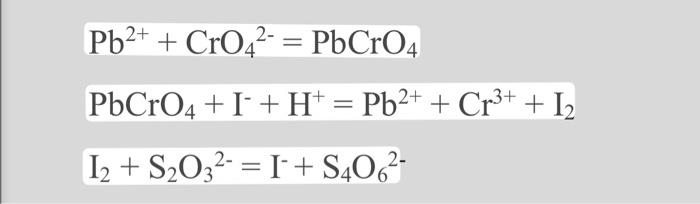
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


