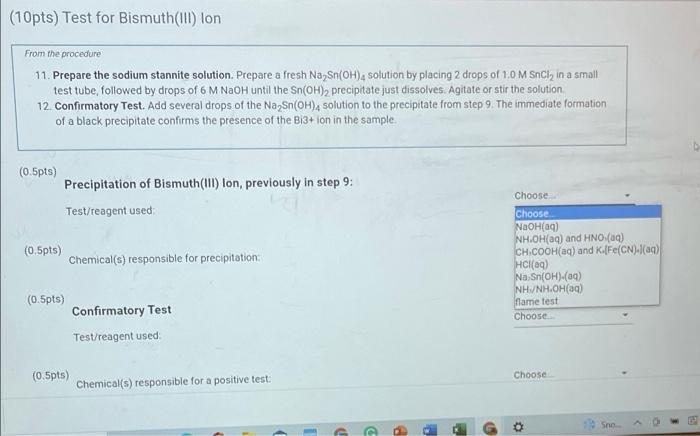
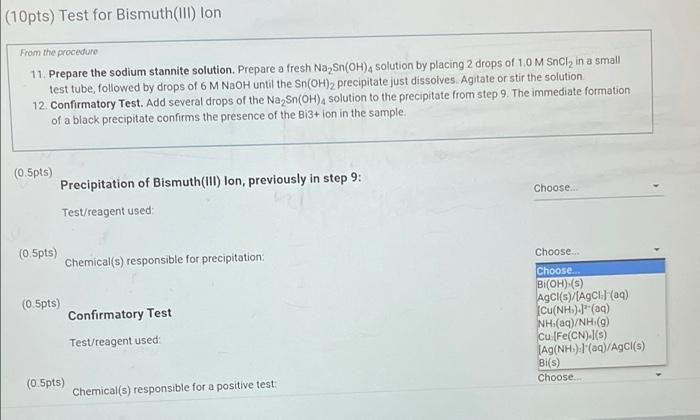
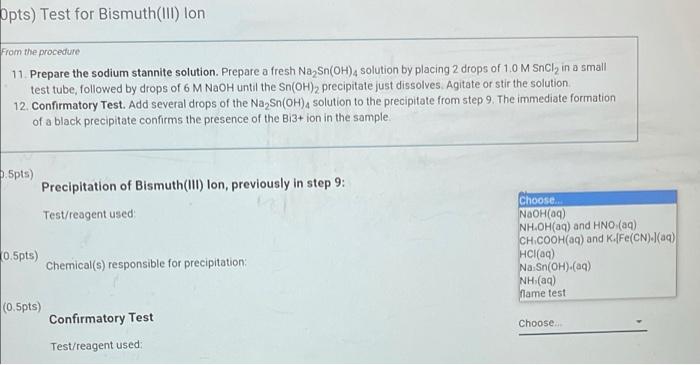
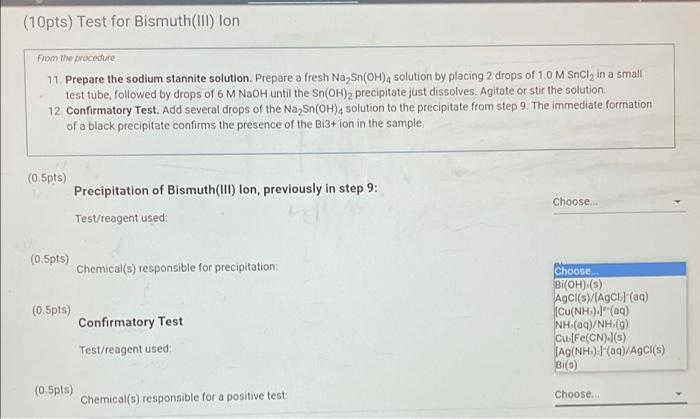
(10pts) Test for Bismuth(III) lon From the procedure 11. Prepare the sodium stannite solution. Prepare a fresh Na Sn(OH), solution by placing 2 drops of 1,0 M Sncly in a small test tube followed by drops of 6 M NaOH until the Sn(OH)2 precipitate just dissolves. Agitate or stir the solution 12. Confirmatory Test. Add several drops of the Na Sn(OH), solution to the precipitate from step 9. The immediate formation of a black precipitate confirms the presence of the Bi3+ ion in the sample. (0.5pts) Precipitation of Bismuth(III) Ion, previously in step 9: Test/reagent used (0.5pts) Chemical(s) responsible for precipitation Choose Choose NaOH 9) NH.OH(aq) and HNO (19) CH.COOH(aq) and K.[Fe(CN)/(aq) HCl(aq) Na Sn(OH)-(09) NH/NH.OH(aq) flame test Choose (0.5pts) Confirmatory Test Test/reagent used (0.5pts) Choose Chemical(s) responsible for a positive test D P . Sno G o (10pts) Test for Bismuth(111) lon From the procedure 11. Prepare the sodium stannite solution. Prepare a fresh Na Sn(OH), solution by placing 2 drops of 1.0 M Sncly in a small test tube, followed by drops of 6 M NaOH until the Sn(OH)2 precipitate just dissolves. Agitate or stir the solution 12. Confirmatory Test. Add several drops of the Na Sn(OH), solution to the precipitate from step 9. The immediate formation of a black precipitate confirms the presence of the Bi3+ion in the sample (0.5pts) Choose.. Precipitation of Bismuth(III) lon, previously in step 9: Test/reagent used (0.5pts) Choose.. Chemical(s) responsible for precipitation: (0.5pts) Confirmatory Test Test/reagent used Choose... Bi(OH) (s) AgCl(s)/(AgCla) Cu(NH).(a) NH (aq)/NH (g) Cu [Fe(CN).(5) JAG(NH). I'(aq)/AgCl(s) Bi(S) Choose. (0.5pts) Chemical(s) responsible for a positive test: Opts) Test for Bismuth(III) Ion From the procedure 11. Prepare the sodium stannite solution. Prepare a fresh Na Sn(OH)4 solution by placing 2 drops of 1.0 M SnCl, in a small test tube, followed by drops of 6 M NaOH until the Sn(OH)2 precipitate just dissolves Agitate or stir the solution 12. Confirmatory Test. Add several drops of the Na Sn(OH), solution to the precipitate from step 9. The immediate formation of a black precipitate confirms the presence of the B13+ ion in the sample 5.5pts) Precipitation of Bismuth(III) lon, previously in step 9: Test/reagent used Choose NaOH(09) NH.OH(aq) and HNO (9) CH.COOH(aq) and K.[Fe(CN).(aq) HCl(aq) Na Sn(OH).(aq) NH(aq) Name test 0.5pts) Chemical(s) responsible for precipitation (0.5pts) Confirmatory Test Choose Test/reagent used (10pts) Test for Bismuth(III) Ion From the procedure 11. Prepare the sodium stannite solution. Prepare a fresh Na Sn(OH), solution by placing 2 drops of 1.0 M Sncly in a small test tube, followed by drops of 6 M NaOH until the Sn(OH)2 precipitate just dissolves. Agitate or stir the solution 12. Confirmatory Test. Add several drops of the Na Sn(OH)4 solution to the precipitate from step 9. The immediate formation of a black precipitate confirms the presence of the Bi3+ ion in the sample (0.5pts) Precipitation of Bismuth(111) lon, previously in step 9: Choose. Test/reagent used: (0.5pts) Chemical(s) responsible for precipitation; (0.5pts) Confirmatory Test Test/reagent used Choose BI(OH) (5) AgCl(s)/AgCl) (89) [Cu(NH3)4](aq) NH (aq)/NH (9) cu [Fe(CN) (5) Ag(NH) (aq)/AgCl(s) BI(C) (0.5pts) Chemical(s) responsible for a positive test: Choose