Question
11 13 15 17 16 18 20 24 32 1. An atom of oxygen with a mass number of 17 and an atomic number
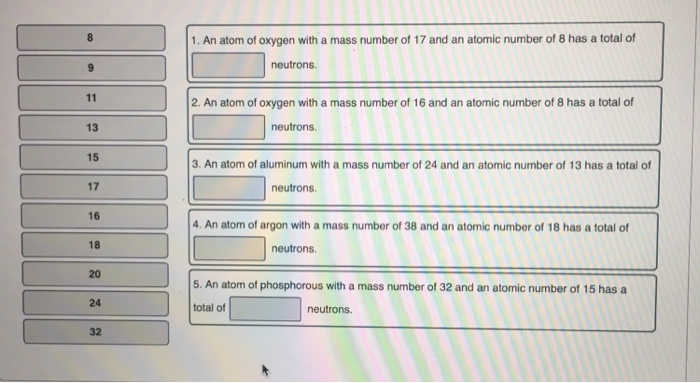
11 13 15 17 16 18 20 24 32 1. An atom of oxygen with a mass number of 17 and an atomic number of 8 has a total of neutrons. 2. An atom of oxygen with a mass number of 16 and an atomic number of 8 has a total of neutrons. 3. An atom of aluminum with a mass number of 24 and an atomic number of 13 has a total of neutrons. 4. An atom of argon with a mass number of 38 and an atomic number of 18 has a total of neutrons. 5. An atom of phosphorous with a mass number of 32 and an atomic number of 15 has a total of neutrons.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below answers are all correct Heres ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Linear Algebra
Authors: Jim Hefferon
1st Edition
978-0982406212, 0982406215
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



