Question
11.13 Find m(H3O+) in a 0.10 mol/kg solution of NaCHO in water at 25C, given that Ka == 1.75 x 10-5 mol/kg for HC2H3O2
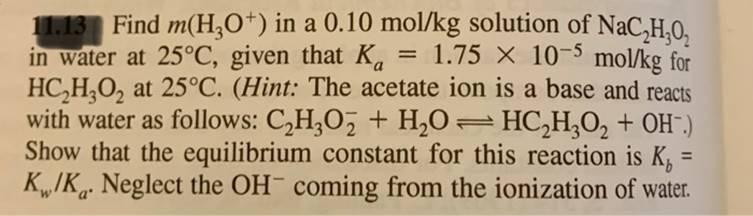
11.13 Find m(H3O+) in a 0.10 mol/kg solution of NaCHO in water at 25C, given that Ka == 1.75 x 10-5 mol/kg for HC2H3O2 at 25C. (Hint: The acetate ion is a base and reacts with water as follows: C2H3O2 + H2O = HC2H3O2 + OH.) Show that the equilibrium constant for this reaction is K = Kw/K. Neglect the OH coming from the ionization of water.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Okay lets solve this stepbystep 1 The reaction that is t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Conceptual Physical Science
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
6th edition
013408229X, 978-0134082295, 9780134080512 , 978-0134060491
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



