Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In this experiment, it will study the reaction between crystal violet and sodium hydroxide. One objective is to study the relationship between concentration of

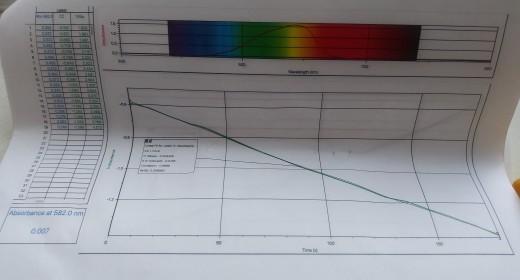




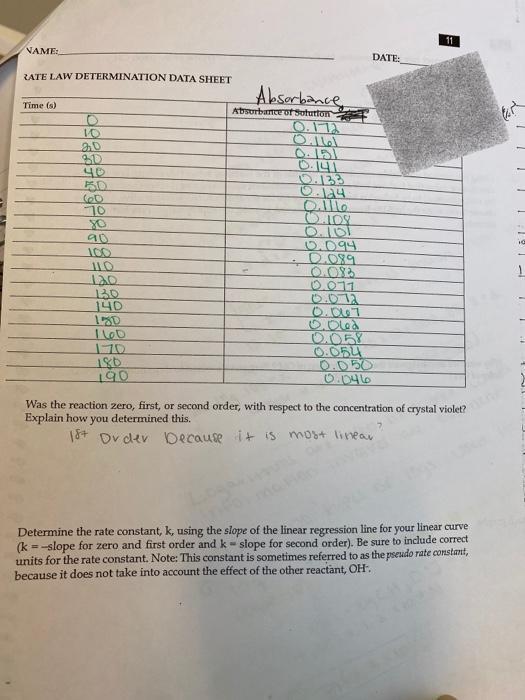
In this experiment, it will study the reaction between crystal violet and sodium hydroxide. One objective is to study the relationship between concentration of crystal violet and the time elapsed during the reaction. The equation for the reaction is shown here. NICH32 NICH3)2 OH (ON(CH)2 + OH N(CH3)2 N(CH)2 N(CH3)2 A simplified (and less intimidating!) version of the equation is: CV" (aq) + OHT (aq) - CVOH (aq) (erystal violet) (hydroxide) The rate law for this reaction is in the form: rate =A[CV'"[OH ". where k is the rate constant for the reaction, m is the order with respect to crystal violet (CV), and n is the order with respect to the hydroxide ion. Because the hydroxide ion concentration is more than 1000 times as large as the concentration of crystal violet, [OH-] will not change appreciably during this experiment. Thus, you will find the order with respect to crystal violet (m), but not the order with respect to hydroxide (n). As the reaction proceeds, a violet-colored reactant will be slowly changing to a colorless product. You will measure the color change with a Vernier Spectrometer. The crystal violet solution used in this experiment has a violet color. Using a Spectrometer it will determine the appropriate wavelength based on the absorbance spectrum of the solution. We will assume that absorbance is proportional to the concentration of crystal violet (Beer's law). Absorbance will be used in place of concentration in plotting the following three graphs: Absorbance vs. time: A linear plot indicates a zero order reaction (k = -slope). In Absorbance vs. time: A linear plot indicates a first order reaction (k = -slope). 1/Absorbance vs. time: A linear plot indicates a second order reaction (k = slope). Once the order with respect to crystal violet has been determined, you will also be finding the rate constant, k. and the half-life for this reaction. !! !! Ti PROCESSING THE DATA 1. Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain. 2. Calculate the rate constant, k, using the slope of the linear regression line for your linear curve (k= - . slope for zero and first order and k= slope for second order). Be sure to include correct units for the rate constant. Note: This constant is sometimes referred to as the pseudo rate constant, because it does not take into account the effect of the other reactant, OH. 3. Write the correct rate law expression for the reaction, in terms of erystal violet (omit OH'). Using the printed data table, estimate the half-life of the reaction; select two points, one with an absorbance value that is about half of the other absorbance value. The time it takes the absorbance (or concentration) to be halved is known the half-life for the reaction. (As an alternative, you may choose to calculate the half-life from the rate constant, k, using the appropriate concentration-time formula.) 4. Linear Fit for: Latest | In Absorbance CC = mt+b %3D m (Slope):-0.004459 b(Y-Intercept): -0.5795 Correlation: -0.9996 RMSE: 0.006990 Write the correct rate law expression for the reaction, in terms of crystal violet (omit OH). 3. vate- KECVI Ising the printed data table, estimate the half-life of the reaction; select two points, one with an 4. absorbance value that is about half of the other absorbance value. The time it takes the absorbance for concentration) to be halved is known the half-life for the reaction. (As an alternative, you may choose to calculate the half-life from the rate constant, k, using the appropriate concentration-time formula.) Why were we able to ignore the concentration of OH? Suggest a method to determine the pseudo order of OH? 5. Attach graphs. 11 NAME: DATE: RATE LAW DETERMINATION DATA SHEET Absorbance, Aborbance ot Sotutton Time (s) 10 30 40 O.151 0.141 D.133 70 O.108 O.101 0.094 - D.089 90 100 0.017 0.072 0.007 140 I le0 170 180 O.058 0.054 0.050 0.046 Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain how you determined this. at Dv dev Decause i+ is most linear Determine the rate constant, k, using the slope of the linear regression line for your linear curve (k = -slope for zero and first order and k- slope for second order). Be sure to include correct units for the rate constant. Note: This constant is sometimes referred to as the pseudo rate constant, because it does not take into account the effect of the other reactant, OH.
Step by Step Solution
★★★★★
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
1 Data Graph time min absorbance ln absorbance 1absorbance 013 0205 15847453 487804878 098 0197 1624...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


