Question
12. Which of the following is NOT a conjugate acid-base pair? a. d. e. NHANH2 H30/H0 HO = H2SO3/HSO3 HSO3 = |* + HSO3-
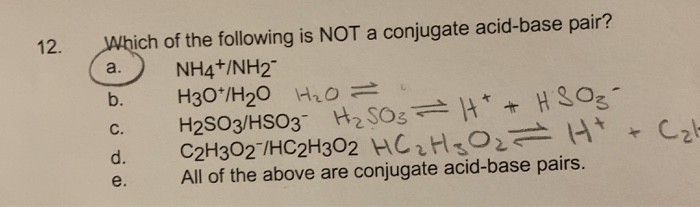
12. Which of the following is NOT a conjugate acid-base pair? a. d. e. NHANH2 H30/H0 HO = H2SO3/HSO3 HSO3 = |* + HSO3- C2H302/HC2H302 HC H 30= H+ All of the above are conjugate acid-base pairs. Cat
Step by Step Solution
3.37 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Answer 12 Answer a NH4 NH NH4 NH is not a conjugate acid base pair since this pair is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2015
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven Gill
33rd Edition
9781305177772, 128543952X, 1305177770, 978-1285439525
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



