Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1200 A 1000 - Liquid a +L C B+L 800 779C (Te) B 8.0 (cas) 91.2 G B Temperature (C) 71.9 (C) (Cee) 600 a
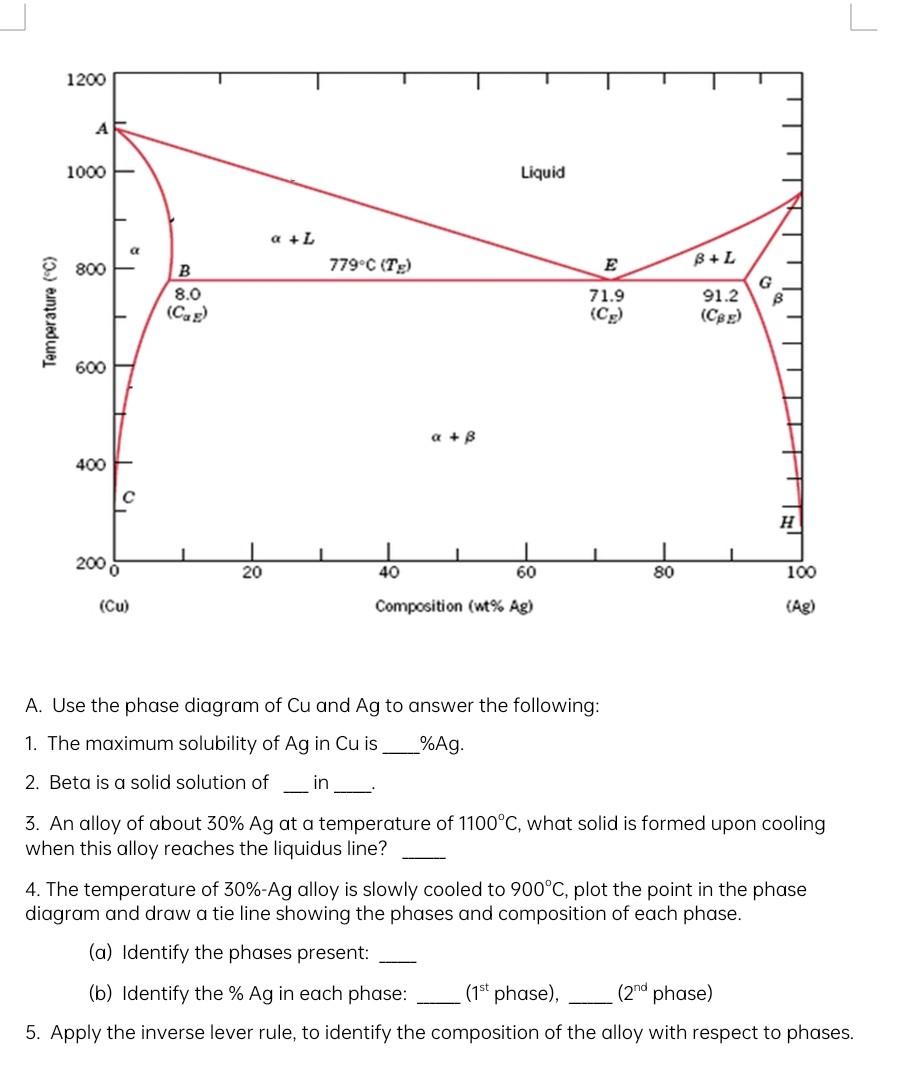
1200 A 1000 - Liquid a +L C B+L 800 779C (Te) B 8.0 (cas) 91.2 G B Temperature (C) 71.9 (C) (Cee) 600 a + 400 H 1 40 1 1 2000 20 60 80 100 (Cu) Composition (wt% Ag) (Ag) A. Use the phase diagram of Cu and Ag to answer the following: 1. The maximum solubility of Ag in Cuis %Ag. 2. Beta is a solid solution of in 3. An alloy of about 30% Ag at a temperature of 1100C, what solid is formed upon cooling when this alloy reaches the liquidus line? 4. The temperature of 30%-Ag alloy is slowly cooled to 900C, plot the point in the phase diagram and draw a tie line showing the phases and composition of each phase. (a) Identify the phases present: (b) Identify the % Ag in each phase: (1st phase), (2nd phase) 5. Apply the inverse lever rule, to identify the composition of the alloy with respect to phases
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


