Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(12.00 Puanlar) 1- CaCO3(s) Cao(s) + CO2(g) What volume of CO2 gas at 0.850 atm and 800 K could be produced by the reaction of
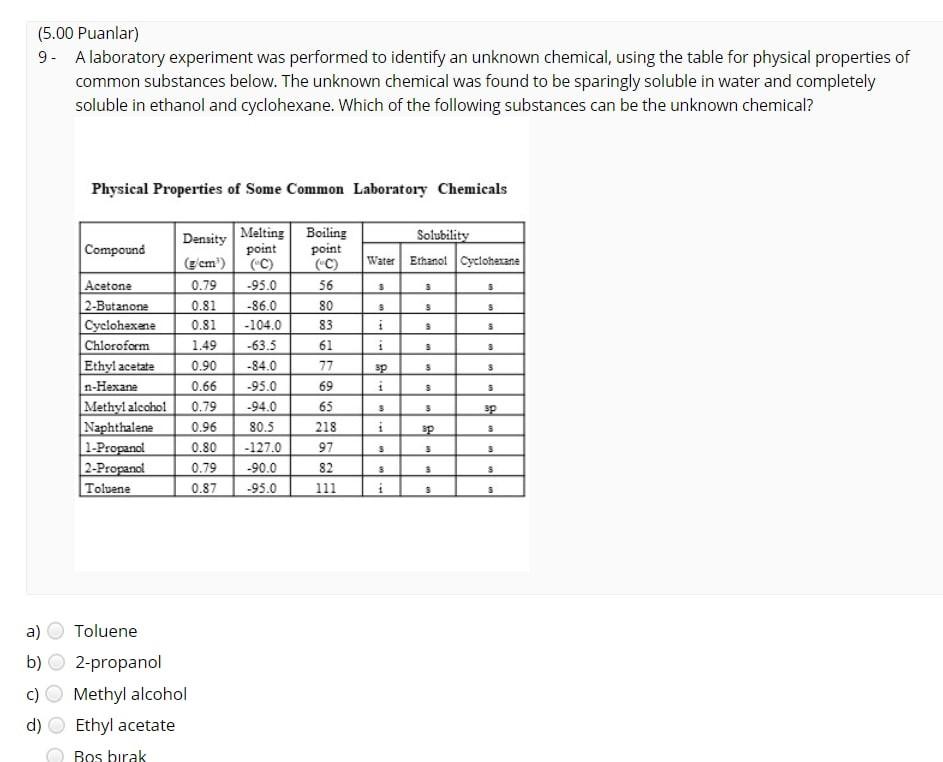
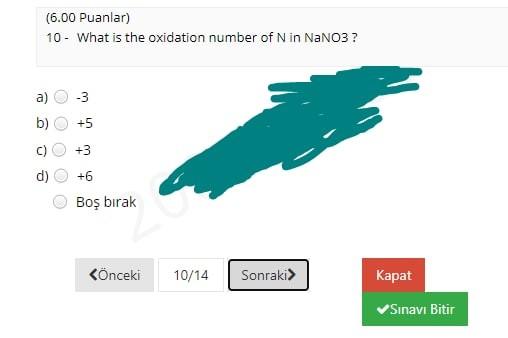
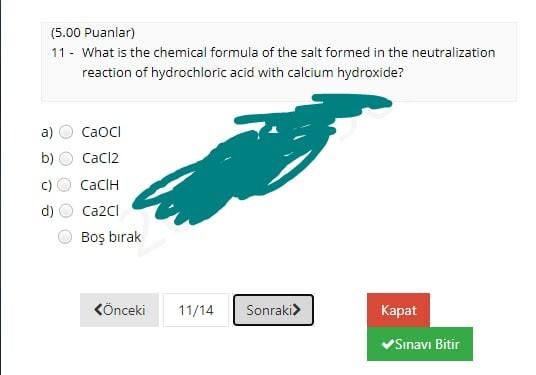
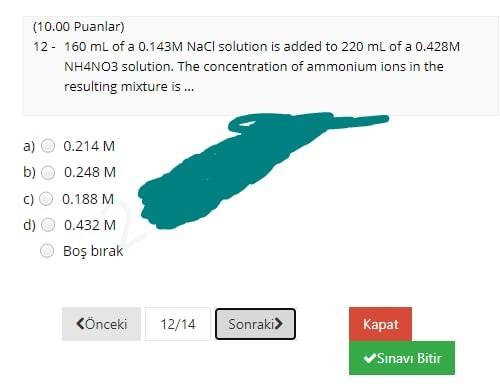
(12.00 Puanlar) 1- CaCO3(s) Cao(s) + CO2(g) What volume of CO2 gas at 0.850 atm and 800 K could be produced by the reaction of 25 g of CaCO3 according to the equation? a) 30.4 L b) 19.3 L 34.7L d) 24.7L Bo brak Knceki 1/14 Sonraki Kapat Sinavi Bitir (4.00 Puanlar) 2 - How many significant figures are present in the result of (6.58030 x 7.91)? a) 2. b) 3 d) 5 Bo brak Knceki 2/14 Sonraki Kapat Sinav Bitir (8.00 Puanlar) 3. In a titration procedure, 60 ml HCl solution is required to neutralize 80 mL of 0.45M NaOH solution. What is the concentration of HCI solution? a) 0.25M b) 1.OM 0.60M d) 1.25M Bo brak Knceki 3/14 Sonraki Kapat Sinavi Bitir (12.00 Puanlar) 4. When 20.0 mL of a 0.250 M (NH4)2S solution is added to 250.0 mL of a solution of Cu(NO3)2, a Cus precipitate forms. If the weight of the precipitate Cus is measured as 0.349 g, what was the concentration of copper ions in the original Cu(NO3)2 solution? a) 0.0194 M C) 0.0205 M 0.0243 M 0.0146 M Bo brak d) Knceki 4/14 Sonraki> Kapat Snav Bitir (7.00 Puanlar) 5. __HCI + __KMnO4- _C12 +_ MnO2 _ _ H2O + _KCI What is the coefficient of KMnO4 when the given equation is properly balanced with the smallest set of whole numbers? a) 00 b) N 4 c) d) 3 3 Bo brak (nceki 5/14 Sonraki Kapat Sinavi Bitir (8.00 Puanlar) 6 - 4NH3 + 3Ca(CIO)2 2N2 + 6H2O + 3CaC12 In the given redox reaction, which element is reduced? N a) ) H CI c) ) O Bo brak Knceki 6/14 Sonraki Kapat Sinavi Bitir (5.00 Puanlar) 7. Which one of the following is the correct molecular equation for the reaction that occurs when solutions of Pb(NO3)2 and Kl are mixed? a) Pb(NO3)2(aq) + Kl(aq) Pb12(s) + KNO3(aq) b) Pb(NO3)2(aq) + 2Kl(aq) Pb12(s) + 2KNO3(aq) Pb2+(aq) + 2NO3- (aq) + 2K+(aq) + 21-(aq) 2K+(aq) + 2NO3- (aq) + Pb12(5) d) Pb2+(aq) + 21- (aq) - Pb12(5) Bo brak Knceki 7/14 Sonraki Kapat Sinavi Bitir (4.00 Puanlar) 8. Which of the following is a chemical property of tin? a) b) Tin melts at 231.9C. Tin can be hammered into a thin sheet Tin erodes when added to hydrochloric acid, and a clear gas forms. Tin is a silvery-white metal. Bo brak d) Knceki 8/14 Sonraki Kapat Sinavi Bitir (5.00 Puanlar) 9. A laboratory experiment was performed to identify an unknown chemical, using the table for physical properties of common substances below. The unknown chemical was found to be sparingly soluble in water and completely soluble in ethanol and cyclohexane. Which of the following substances can be the unknown chemical? Physical Properties of Some Common Laboratory Chemicals Compound Boiling point Solubility Ethanol Cyclohexane Water 3 3 0.81 $ $ $ 1 9 1 Density Melting point (g/cm) (C) 0.79 -95.0 -86.0 0.81 -104.0 1.49 -63.5 0.90 -84.0 0.66 -95.0 0.79 -94.0 0.96 80.5 0.80 -127.0 0.79 -90.0 0.87 -95.0 Acetone 2-Butanone Cyclohexene Chloroform Ethyl acetate n-Hexane Methyl alcohol Naphthalene 1-Propanol 2-Propanol Toluene 5 56 80 83 61 77 69 65 218 97 82 111 sp i $ 5 SP i sp 5 5 3 $ S 3 3 a) Toluene b) 2-propanol c) Methyl alcohol d) Ethyl acetate Bos brak (6.00 Puanlar) 10 - What is the oxidation number of N in NaNO3? a b) c) +5 it to w OOOO d) +6 Bo brak Knceki 10/14 Sonraki> Kapat Snav Bitir (5.00 Puanlar) 11 - What is the chemical formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium hydroxide? a) b) CaOCI CaCl2 Cac Ca2cl Bo brak d) Knceki 11/14 Sonraki Kapat Sinav Bitir (10.00 Puanlar) 12 - 160 mL of a 0.143M NaCl solution is added to 220 mL of a 0.428M NH4NO3 solution. The concentration of ammonium ions in the resulting mixture is ... a) 0.214 M b) 0.248 M 0.188 M 0.432 M Bo brak Knceki 12/14 Sonraki Kapat Sinavi Bitir (10.00 Puanlar) 13- Calculate the mass of 2.47 L of CO gas measured at 33C and 924 mmHg. a) b) 3.358 3.78 g 3.178 3.57 8 c) d) Bo brak Knceki 13/14 Sonraki Kapat Sinavi Bitir (4.00 Puanlar) 14. Which of the following is a pure substance? Soup a) b) Steam d) Orange Juice Air Bo brak Phone Knceki 14/14 Sonraki Kapat Snav Bitir
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


