Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13. 14. 15. The gure shows a p V-diagram for 0.0040 mole of hydrogen gas. The temperature of the gas does not change during segment
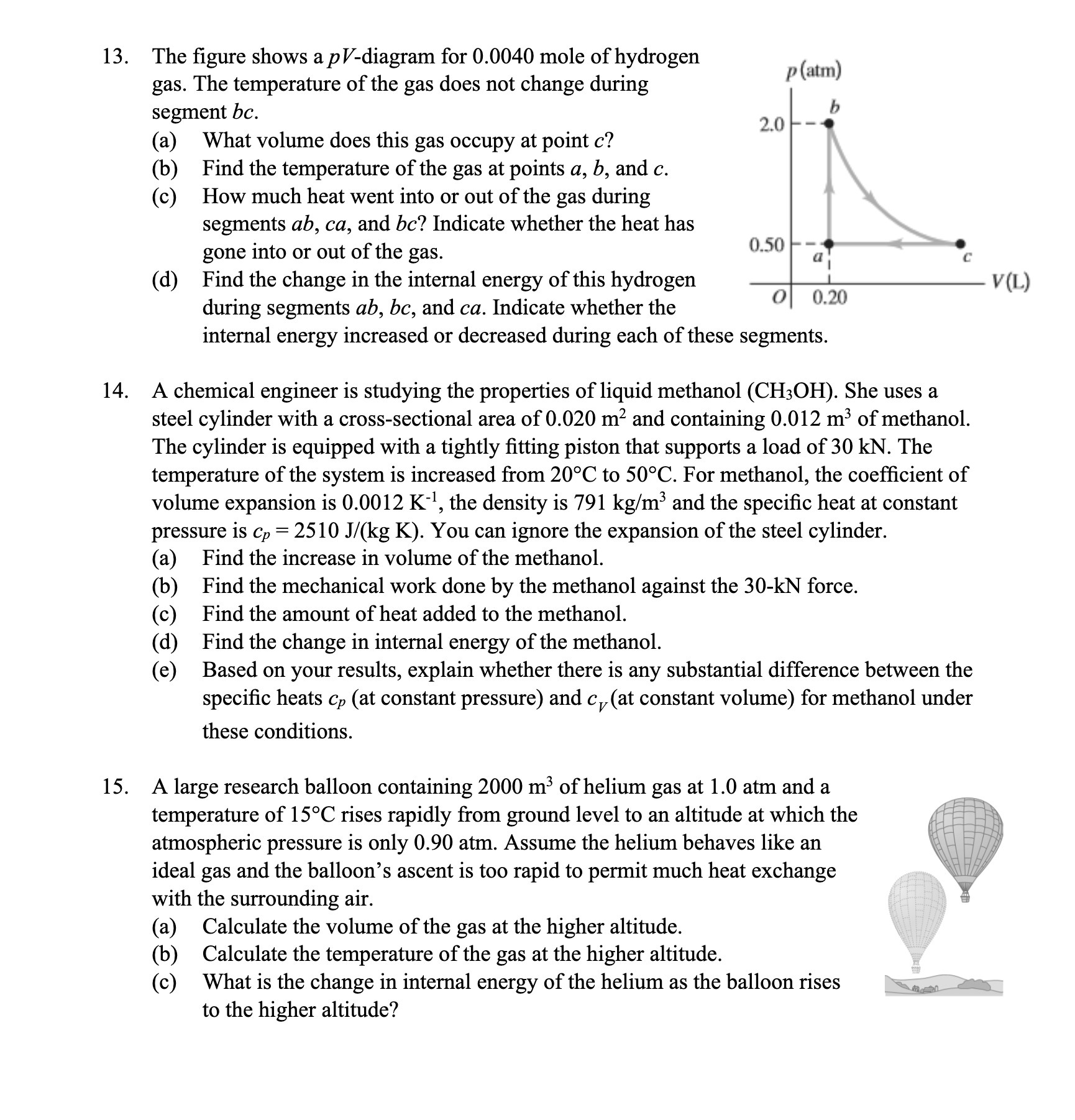 13. 14. 15. The gure shows a p V-diagram for 0.0040 mole of hydrogen gas. The temperature of the gas does not change during segment bc. (a) What volume does this gas occupy at point c? (b) Find the temperature of the gas at points a, b, and c. (c) How much heat went into or out of the gas during segments ab, ca, and bc? Indicate whether the heat has gone into or out of the gas. ((1) Find the change in the internal energy of this hydrogen during segments ab, bc, and ca. Indicate whether the internal energy increased or decreased during each of these segments. A chemical engineer is studying the properties of liquid methanol (CH30H). She uses a steel cylinder with a cross-sectional area of 0.020 m2 and containing 0.012 m3 of methanol. The cylinder is equipped with a tightly tting piston that supports a load of 30 kN. The temperature of the system is increased from 20C to 50C. For methanol, the coefcient of volume expansion is 0.0012 K], the density is 791 kg/m3 and the specic heat at constant pressure is cp = 2510 J/(kg K). You can ignore the expansion of the steel cylinder. (a) Find the increase in volume of the methanol. (b) Find the mechanical work done by the methanol against the 30-kN force. (0) Find the amount of heat added to the methanol. ((1) Find the change in internal energy of the methanol. (6) Based on your results, explain whether there is any substantial difference between the specic heats 6,; (at constant pressure) and cV(at constant volume) for methanol under these conditions. A large research balloon containing 2000 In3 of helium gas at 1.0 atm and a temperature of 15C rises rapidly from ground level to an altitude at which the atmospheric pressure is only 0.90 atm. Assume the helium behaves like an ideal gas and the balloon's ascent is too rapid to permit much heat exchange with the surrounding air. (a) Calculate the volume of the gas at the higher altitude. (b) Calculate the temperature of the gas at the higher altitude. : (c) What is the change in internal energy of the helium as the balloon rises M to the higher altitude
13. 14. 15. The gure shows a p V-diagram for 0.0040 mole of hydrogen gas. The temperature of the gas does not change during segment bc. (a) What volume does this gas occupy at point c? (b) Find the temperature of the gas at points a, b, and c. (c) How much heat went into or out of the gas during segments ab, ca, and bc? Indicate whether the heat has gone into or out of the gas. ((1) Find the change in the internal energy of this hydrogen during segments ab, bc, and ca. Indicate whether the internal energy increased or decreased during each of these segments. A chemical engineer is studying the properties of liquid methanol (CH30H). She uses a steel cylinder with a cross-sectional area of 0.020 m2 and containing 0.012 m3 of methanol. The cylinder is equipped with a tightly tting piston that supports a load of 30 kN. The temperature of the system is increased from 20C to 50C. For methanol, the coefcient of volume expansion is 0.0012 K], the density is 791 kg/m3 and the specic heat at constant pressure is cp = 2510 J/(kg K). You can ignore the expansion of the steel cylinder. (a) Find the increase in volume of the methanol. (b) Find the mechanical work done by the methanol against the 30-kN force. (0) Find the amount of heat added to the methanol. ((1) Find the change in internal energy of the methanol. (6) Based on your results, explain whether there is any substantial difference between the specic heats 6,; (at constant pressure) and cV(at constant volume) for methanol under these conditions. A large research balloon containing 2000 In3 of helium gas at 1.0 atm and a temperature of 15C rises rapidly from ground level to an altitude at which the atmospheric pressure is only 0.90 atm. Assume the helium behaves like an ideal gas and the balloon's ascent is too rapid to permit much heat exchange with the surrounding air. (a) Calculate the volume of the gas at the higher altitude. (b) Calculate the temperature of the gas at the higher altitude. : (c) What is the change in internal energy of the helium as the balloon rises M to the higher altitude

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


