Answered step by step
Verified Expert Solution
Question
1 Approved Answer
13 parts a and b Consider the following reaction where Kc=6.30 at 723K. 2NH3(g)N2(g)+3H2(g) A reaction mixture was found to contain 0.000732 moles of NH3(g),0.0298
13 parts a and b 
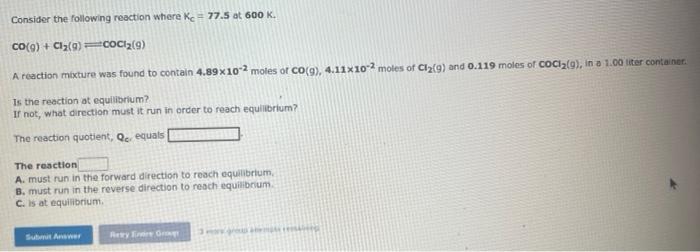
Consider the following reaction where Kc=6.30 at 723K. 2NH3(g)N2(g)+3H2(g) A reaction mixture was found to contain 0.000732 moles of NH3(g),0.0298 moles of N2(g), and 0.0447 moles of H2(g), in a 1.00 iter contaner. calculate Qc. Qa= Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reection must run in the forward direction to reach equillbrium. The reaction must run in the reverse direction to reach equilibrlum. The reaction is at equtibrium. Consider the following reaction where Kc=77.5 at 600K. CO(9)+Cl2(g)cocl2(9) A reaction mixture was found to contain 4.89102 moles of Co(9),4.11102 moles of Cl2(9) and 0.119 moles of coCl(9), in a 1.00 iter container. Th. the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qerequals The resction A. must run in the forward difection to reach equilibriurn. B. must run in the reverse direction to reach equilibrum. C. is at equilibrium 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


